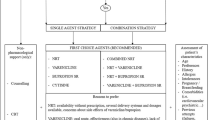
Abstract
Smoking cessation presents a daunting challenge for clinicians; tobacco use impacts every major organ system and is responsible for considerable morbidity and mortality. Despite significant reductions in the rate of smoking over the past 50 years, tobacco use continues to burden the healthcare system. First-line therapies for smoking cessation include nicotine replacement, novel partial nicotinic agonists as well as antidepressant therapy. Herein, we review recent updates to the literature regarding nicotine replacement, bupropion, and varenicline. Re-emerging therapies such as cytisine are reviewed, as well as the novel concept of vaccination. Finally, the controversy surrounding electronic cigarettes and their debatable role in cessation therapy will be addressed as their popularity continues to grow.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
The health consequences of smoking—50 years of progress: a report of the Surgeon General. 2014: U.S. Department of Health and Human Services.
Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. U.S. Department of Health and Human Services, Public Health Service 2008; Available from: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf.
Aubin HJ, Luquiens A, Berlin I. Pharmacotherapy for smoking cessation: pharmacological principles and clinical practice. Br J Clin Pharmacol. 2014;77(2):324–36.
Rennard SI, Daughton DM. Smoking cessation. Clin Chest Med. 2014;35(1):165–76.
Stead LF et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146.
Brokowski L, Chen J, Tanner S. High-dose transdermal nicotine replacement for tobacco cessation. Am J Health Syst Pharm. 2014;71(8):634–8.
Tonnesen P et al. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. Eur Respir J. 2012;40(3):548–54.
Shahab L, Brose LS, West R. Novel delivery systems for nicotine replacement therapy as an aid to smoking cessation and for harm reduction: rationale, and evidence for advantages over existing systems. CNS Drugs. 2013;27(12):1007–19.
Schnoll RA et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175(4):504–11.
Hughes JR et al. Are higher doses of nicotine replacement more effective for smoking cessation? Nicotine Tob Res. 1999;1(2):169–74.
Schnoll RA et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152(3):144–51.
Hansson A, Hajek P, Perfekt R, et al. Effects of nicotine mouth spray on urges to smoke, a randomised clinical trial. BMJ Open 2012;2:e001618. doi:10.1136/bmjopen-2012-001618.
Caldwell BO, Adamson SJ, Crane J. Combination rapid-acting nicotine mouth spray and nicotine patch therapy in smoking cessation. Nicotine Tob Res. 2014;16(10):1356–64.
Gonzales D et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55.
Jorenby DE et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63.
Hurt RD et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337(17):1195–202.
Durcan MJ et al. The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clin Ther. 2002;24(4):540–51.
Hughes JR et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031.
Ebbert JO et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311(2):155–63.
Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. Am J Psychiatry. 2014;171(11):1199–205.
Coe JW et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–7.
Hays JT, Ebbert JO. Varenicline for tobacco dependence. N Engl J Med. 2008;359(19):2018–24.
Ebbert JO et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687–94.
Rigotti NA et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221–9.
Singh S et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183(12):1359–66.
Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
Ware JH et al. Cardiovascular safety of varenicline: patient-level meta-analysis of randomized, blinded, placebo-controlled trials. Am J Ther. 2013;20(3):235–46.
Mills EJ et al. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28–41. Large meta-analysis examining the existing evidence regarding potential cardiovascular adverse effects of varenicline use for smoking cessation following FDA post-marketing reports of increased events.
Moore TJ et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6(11):e27016.
Gibbons RD, Mann JJ. Varenicline, smoking cessation, and neuropsychiatric adverse events. Am J Psychiatry. 2013;170(12):1460–7.
FDA Drug Safety Communication: FDA updates label for stop smoking drug Chantix (varenicline) to include potential alcohol interaction, rare risk of seizures, and studies of side effects on mood, behavior, or thinking. 2015 March 9, 2015 [cited 2015 June 1]; Available from: http://www.fda.gov/Drugs/DrugSafety/ucm436494.htm.
Thomas KH et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
Ahmed AI et al. Neuropsychiatric adverse events of varenicline: a systematic review of published reports. J Clin Psychopharmacol. 2013;33(1):55–62.
Meyer TE et al. Neuropsychiatric events in varenicline and nicotine replacement patch users in the Military Health System. Addiction. 2013;108(1):203–10.
West R et al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med. 2011;365(13):1193–200.
Walker N et al. Cytisine versus nicotine for smoking cessation. N Engl J Med. 2014;371(25):2353–62. An open label trial of 1310 patients to try to establish non-inferiority of cytisine to traditional NRT. It examined self-reported quit rates at 1 month.
Prochaska JJ, Das S, Benowitz NL. Cytisine, the world’s oldest smoking cessation aid. BMJ. 2013;347:f5198.
Yamin CK, Bitton A, Bates DW. E-cigarettes: a rapidly growing Internet phenomenon. Ann Intern Med. 2010;153(9):607–9.
Bhatnagar A et al. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418–36.
McCarthy M. “Alarming” rise in popularity of e-cigarettes is seen among US teenagers as use triples in a year. BMJ. 2015;350:h2083.
Ebbert JO, Agunwamba AA, Rutten LJ. Counseling patients on the use of electronic cigarettes. Mayo Clin Proc. 2015;90(1):128–34.
Bartter T. Electronic cigarettes: aggregate harm. Ann Intern Med. 2015;163(1):59–60.
Deeming – Extending Authorities to Additional Tobacco Products. 2/26/2015; Available from: http://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm388395.htm.
Jensen RP et al. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–4.
Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493–4.
Orr MS. Electronic cigarettes in the USA: a summary of available toxicology data and suggestions for the future. Tob Control. 2014;23 Suppl 2:ii18–22.
Bullen C et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–37. Randomized controlled superiority trial comparing electronic cigarettes to placebo electronic cigarettes versus nicotine patches. Abstinence rates were examined 6 months following intervention.
Caponnetto P et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317.
McRobbie H et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:CD010216. An important examination of the two RCTs and cohort studies currently available reporting on the possible efficacy of electronic cigarettes as a smoking cessation tool.
Cornuz J et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3(6):e2547.
Raupach T, Hoogsteder PH, Onno van Schayck CP. Nicotine vaccines to assist with smoking cessation: current status of research. Drugs. 2012;72(4):e1–e16.
Hoogsteder PH et al. Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial. Addiction. 2014;109(8):1252–9.
Koegelenberg CF et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312(2):155–61.
Compliance with Ethics Guidelines
Conflict of Interest
Drs Allam and Ochoa both state they have no conflicts to disclose.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Smoking Cessation
Rights and permissions
About this article
Cite this article
Allam, J.S., Ochoa, C.D. Pharmacological therapies in smoking cessation: an evidence-based update. Curr Pulmonol Rep 4, 173–178 (2015). https://doi.org/10.1007/s13665-015-0125-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-015-0125-5