Abstract
Three new ent-kaurane diterpenoids, silvaticusins A–C (1–3), along with a new ent-kaurane dimer silvaticusin D (4) were isolated from the aerial parts of Isodon silvaticus. The structures of these new compounds were established mainly by comprehensive analysis of their NMR and MS data. The absolute configuration of compounds 1 and 4 were determined using a single-crystal X-ray diffraction and computational methods, respectively. Compounds 2 and 3 were found to exhibit remarkable cytotoxic effects against five human tumor cell lines (HL-60, A-549, SMMC-7721, MDA-MB-231, and SW-480), with IC50 values spanning from 1.27 ± 0.08 to 7.52 ± 0.33 μM.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Isodon species, highly esteemed for their medicinal virtues, harbor a rich array of diterpenoids, with ent-kauranoids being the most prominent class [1, 2]. The recent discovery of labdane-based meroditerpenoids with cyclobutane moieties, including isoscopariusins B and C [3], along with clerodane-based dimers scospirosins A and B [4], and scoparicacids A–C [5] from I. scoparius, further highlights the chemical versatility of these plants. Furthermore, the bioactivities of these compounds are particularly notable. For example, eriocalyxin B, derived from I. eriocalyx var. laxiflora, has recently been found to demonstrate significant therapeutic potential in oncology by effectively inhibiting cell migration in triple-negative breast cancer [6].
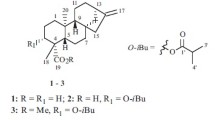
In a concerted effort to uncover natural products with distinctive structures and potent bioactivities from the genus Isodon, a comprehensive phytochemical study was conducted on Isodon silvaticus collected from Changdu in the Tibet Autonomous Region. To date, there has been a scarcity of information regarding the chemical composition of I. silvaticus, except that a reported synthetic derivative acetyl-macrocalin B [7] has been isolated from this plant. This compound has undergone an in-depth pharmacological assessment, revealing its remarkable capacity to inhibit the growth of non-small cell lung cancer and esophageal squamous cell carcinoma in both in vitro and in vivo models [8, 9]. The current study resulted in the isolation of silvaticusins A–D (1–4) (Fig. 1), involving two new C-20-oxygenated-ent-kaurane diterpenoids (1 and 2), a new C-20 non-oxygenated-ent-kaurane diterpenoid (3), and a new dimeric diterpenoid (4) characterized by a cyclobutane moiety formed through a [2 + 2] cycloaddition between two 6,7-seco-ent-kaurane diterpenoids. Meanwhile, 37 miscellaneous known diterpenoids were characterized from the same plant material (Fig. S44 and Table S13). This study presents the first characterization of new compounds from I. silvaticus. Notably, the discovery of silvaticusin D (4) enriches the limited array of ent-kaurane dimers with a cyclobutane ring. This group now includes maoecrystal M (7,20-epoxy-ent-kaurane dimer) [10], bisjaponins A and B (6,7-seco-ent-kaurane dimer, 6,7-seco-/7,20-epoxy-ent-kaurane dimer, respectively) [11], and bistenuifolin L and M (C-20 non-oxygenated ent-kaurane dimers) [12]. The cyclobutane moiety in these compounds, formed through [2 + 2] cycloaddition, is a key structural feature that may confer unique biological properties and synthetic challenges, underscoring their importance in the field of natural product chemistry [13, 14]. The structures of silvaticusins A–D (1–4) were determined through a combinatorial use of spectroscopic analysis, X-ray diffraction, and quantum chemical calculations. Notably, compounds 2 and 3 were found to exhibit remarkable cytotoxic activities against HL-60, A-549, SMMC-7721, MDA-MB-231, and SW-480 human tumor cell lines with IC50 ranging from 1.27 ± 0.08 to 7.52 ± 0.33 μM. Herein, the structure elucidation and bioactivity evaluation of silvaticusins A–D (1–4) were reported.
2 Results and discussion
Silvaticusin A (1), which was obtained as colorless crystals, had the molecular formula C20H30O6 as inferred from the (+)-HRESIMS ion at m/z 389.1936 [M + Na]+ (calcd 389.1535) and supported by 13C NMR data. The IR spectrum exhibited characteristic absorption bands indicative of hydroxy (3391 cm−1), and olefinic (1633 cm−1) functionalities. Its 1H NMR spectrum showed resonances of two methyl groups at δH 1.05 (s) and 1.15 (s), four oxygenated methines at δH 4.21 (d, J = 5.3 Hz), 4.32 (t, J = 8.2 Hz), 5.34 (br s), and 5.72 (br s), and two olefinic protons at δH 5.36 (d, J = 1.9 Hz) and 5.74 (d, J = 1.9 Hz) (Table 1). Its 13C NMR and DEPT spectra featured twenty carbon resonances, including two methyl groups, six methylenes (one olefinic, one oxygenated), seven methines (four oxygenated), five non-protonated carbons (three quaternary) (Table 2). Comprehensive analysis of the 1D and 2D NMR data of 1 manifested that its structure resembled that of the 7,20-epoxy-ent-kauranoid, enmenol [15]. The H2-1/H2-2/H2-3, H-5/H-6, and H-9/H2-11/H-12/H-13/H-14 correlations in the 1H − 1H COSY spectrum, in tandem with the H2-1/C-20, H-6/C-4, H-6/C-7, H-6/C-8 correlations in the HMBC spectrum (Fig. 2) indicated that silvaticusin A (1) possesses a hydroxyl group at the C-12 position, differing from the C-1 hydroxylation in enmenol, while the rest of their structures were consistent.
As for the configurational determination of 1, assigning H-5 as β-oriented, the 3JH-5/H-6 (5.3 Hz) indicated that H-6 was α-oriented. The H-9/H-5β, H-12/H-9, H3-18/H-5β, H-20b/H3-19, and H-14/H-20a correlations in the ROESY spectrum indicated that H-9, H-12, HO-14 adopted β orientation, while C-20 adopted α orientation. But the orientation of HO-15 could not be conclusively assigned. Fortunately, well-formed crystals of compound 1 were successfully procured using methanol and subsequently underwent X-ray crystallographic analysis. The refined Cu Kα data yielded a Flack parameter of –0.13(15), which enabled the determination of its absolute configuration as 5R, 6S, 7R, 9R, 10R, 12S, 13R, 14R, and 15R (Fig. 3). Thus, the structure of silvaticusin A (1) was defined as 6β,7β,12α,14β,15β-pentahydroxy-7,20-epoxy-ent-kaur-16-ene.
Silvaticusin B (2), which was obtained as a white amorphous powder, had the molecular formula C20H28O5 as established by the ( +)-HRESIMS ion at m/z 349.2010 [M + H]+ (calcd 349.2010). The 1H and 13C NMR data of compound 2 (Tables 1 and 2) were similar to those of 1. The 1H − 1H COSY spectrum exhibited correlations of H2-1/H2-2/H2-3, H-5/H2-6, H-9/H2-11/H-12, and H-13/H-14 (Fig. 2). And the HMBC spectrum displayed correlations from H-5 to C-6, C-7, C-10, and C-19, from H-13 to C-8, C-12, C-14, C-15, C-16 and C-17. These findings demonstrated that compound 2 features a carbonyl at C-15 instead of a hydroxy group as in 1, and a methylene at C-6 instead of the oxygenated methine in 1. Analysis of the ROESY spectrum of 2 uncovered the β orientations of H-12, HO-14. Thus, the structure of silvaticusin B (2) was assigned as 7β,12α,14β-trihydroxy-7,20-epoxy-ent-kaur-16-en-15-one.
Silvaticusin C (3) was isolated as a white amorphous powder. The molecular formula C20H28O5 was deduced from HREIMS and 13C NMR data. A thorough examination of the 1H and 13C NMR data for compound 3 revealed that its structure bore a close resemblance to that of the C-20 non-oxygenated-ent-kaurane diterpenoid macroclyxin C [16], sharing an identical molecular weight of 348 Daltons. The 1H − 1H COSY spectrum, with key correlations of H2-1/H2-2/H-3, H-5/H2-6/H-7, and H-9/H2-11/H2-12/H-13/H-14, and the HMBC spectrum (Fig. 2), showing correlations from CHO-18 to C-3, C-4, and C-19, and from H-13 to C-12 and C-15, have pinpointed a hydroxyl group at C-12 in silvaticusin C (3). This differs from macroclyxin C's hydroxyl group at C-3, with the rest of their structures remains congruent. As for the stereochemistry of 3, appointing H-5 as β-oriented, the H-3/H-5β, H-7/H-5β, H-9/H-5β, CHO-18/H-5β, H3-20/H3-19, H-14/H3-20 correlations in the ROESY spectrum indicated the β orientation of H-3, H-7, CHO-18, and HO-14. Therefore, the structure of silvaticusin C (3) was assigned as 3α,7α,14β-trihydroxy-ent-kaur-16-en-18-al-15-one.
Silvaticusin D (4) was isolated as a white amorphous powder. The molecular formula of C42H55O14 was inferred from the (+)-HRESIMS ion at m/z 789.3458 [M + Na]+ (calcd 789.3457) and 13C NMR data. Detailed analysis of the 1H and 13C NMR data of 4 suggested that it is a diterpene dimer composed of two 6,7-seco-6,20-epoxy-ent-kauranoids analogous to the structures of epinodosin [17, 18] and enmein-3-acetate [19], respectively. This deduction was further supported by H-1/H2-2/H2-3, and H-9/H-11/H2-12/H-13/H-14, H-1′/H2-2′/H-3′, and H-9′/H2-11′/H2-12′/H-13′/H-14′ correlations in the 1H − 1H COSY spectrum, as well as HMBC correlations from H-6 to C-4, C-5, C-10, C-20, from H-11 to C-8, C-10, C-13, from H-6′ to C-4′, C-10′, C-20′, from H-3′ to C-1′, C-2′, C-4′, C-5′, -OAc. The lack of NMR signals for the typical terminal double bonds at C-16/C-17, a feature usually presents in ent-kauranoids, in both monomers of compound 4, implies that these double bonds are involved in the dimerization. This, combined with the one remaining degree of unsaturation and key 1H − 1H COSY correlation between H-17 and H-17′, along with HMBC correlations of H-13/C-16, H2-17/C-13, H-13′/C-16′, and H2-17′/C-13′, supported a head-to-head [2 + 2] cycloaddition mechanism for dimerization. Accordingly, the planar structure of compound 4 is established as illustrated in Fig. 4.
The stereochemistry of most chiral centers was determined using ROESY correlations and coupling constants, except for C-3′, where overlapping NMR signals obscured its configuration determination. As for the two new chiral centers (C-16 and C-16′) formed during dimerization, correlations of H-13/H-17a, H-13/H-17′a, and H-13′/H-17′b in the ROESY spectrum suggested the α orientation of C-17 methylene, and the β orientation of C-17′. Then, the two possible epimers, (1S*, 5R*, 6R*, 8S*, 9S*, 10S*, 11S*, 13S*, 16R*, 1′S*, 3′S*, 5′R*, 6′R*, 8′S*, 9′S*, 10′S*, 13′R*, 16′R*)-4 (4a) and (1S*, 5R*, 6R*, 8S*, 9S*, 10S*, 11S*, 13S*, 16R*, 1′S*, 3′R*, 5′R*, 6′R*, 8′S*, 9'S*, 10′S*, 13′R*, 16′R*)-4 (4b) were subjected to NMR computation at the mPW1PW91-SCRF/6–31 + G(d,p)//B3LYP-D3BJ/6-31G(d) level of theory. In the subsequent DP4 + analysis [20] comparing isomers 4a and 4b, 4a exhibited a conclusive probability of 100% (Figure S46), thereby substantiating its designation as the correct structure. Then, TDDFT ECD calculation of (1S, 5R, 6R, 8S, 9S, 10S, 11S, 13S, 16R, 1′S, 3′S, 5′R, 6′R, 8′S, 9′S, 10′S, 13′R, 16′R)-4 (4A) were carried out at the PBE0-SCRF/6–311 + G(2d,p) and CAM-B3LYP-SCRF/6–311 + G(2d,p) level of theory, respectively, and the calculated curves matched the experimental curve (Fig. 5), thereby determining the absolute configuration of compound 4 as 1S, 5R, 6R, 8S, 9S, 10S, 11S, 13S, 16R, 1′S, 3′S, 5′R, 6′R, 8′S, 9′S, 10′S, 13′R, and 16′R (Table 3).
Previous research on diterpenoids from the Isodon genus has shown that the exo-methylene cyclopentanone D-ring system is crucial for their antitumor and anti-inflammatory effects, with C-14 hydroxylation usually enhancing these effects [21]. In this research, compounds 2 and 3, which possess this D-ring system, were evaluated for cytotoxicity against HL-60, A-549, SMMC-7721, MDA-MB-231, and SW-480 cell lines using the MTS assay [22], with cisplatin and paclitaxel as positive controls. Compounds 2 and 3 exhibited significant cytotoxic activity, with IC50 values ranging from 1.27 ± 0.08 to 7.52 ± 0.33 μM for all five cell lines (Table 4).
3 Experimental
3.1 General experimental procedures
General experimental procedures can be found in the Supplementary Material (Part 1).
3.2 Plant material
The source of the plant material can be found in the Supplementary Material (Part 2).
3.3 Extraction and isolation
The detailed procedures for extraction and isolation can be found in in the Supplementary Material (Part 3).
3.4 Characteristic data of compounds 1–4
Silvaticusin A (1): colorless needles; mp 195 ~ 197 °C; \([\alpha]_{\text{D}}^{20}\) − 16.04 (c 0.106, MeOH); IR (KBr) νmax 3391, 2948, 2929 cm−1; 1H and 13C NMR data, see Tables 1 and 2; positive-ion HREIMS [M + Na]+ m/z 389.1936 (calcd for C20H30O6Na, 389.1935).
Crystallographic data for silvaticusin A (1): C20H30O6•2(H2O), M = 402.47, a = 6.4305(2) Å, b = 16.4111(5) Å, c = 18.9447(5) Å, α = 90°, β = 90°, γ = 90°, V = 1999.26(10) Å3, T = 150.(2) K, space group P212121, Z = 4, μ(Cu Kα) = 0.851 mm−1, 14,239 reflections measured, 3632 independent reflections (Rint = 0.1309). The final R1 values were 0.0415 (I > 2σ(I)). The final wR(F2) values were 0.1046 (I > 2σ(I)). The final R1 values were 0.0639 (all data). The final wR(F2) values were 0.1146 (all data). The goodness of fit on F2 was 1.042. Flack parameter = − 0.13(15).
Silvaticusin B (2): white, amorphous powder; \([\alpha]_{\text{D}}^{20}\) − 48.66 (c 0.06, MeOH); IR (KBr) νmax 3427, 2948, 2928, 1724 cm−1; 1H and 13C NMR data, see Tables 1 and 2; positive-ion HREIMS [M + H]+ m/z 349.2010 (calcd for C20H29O5, 349.2010).
Silvaticusin C (3): white, amorphous powder; \([\alpha]_{\text{D}}^{25}\) − 73.33 (c 0.09, MeOH); 1H and 13C NMR data, see Tables 1 and 2; positive-ion HREIMS [M + H]+ m/z 349.2008 (calcd for C20H29O5, 349.2010).
Silvaticusin D (4): white, amorphous powder; \([\alpha]_{\text{D}}^{25}\) − 22.61 (c 0.092, MeOH); 1H and 13C NMR data, see Table 3; positive-ion HREIMS [M + Na]+ m/z 789.3458 (calcd for C42H55O14Na, 789.3457).
3.5 X-ray crystal structure analysis
The X-ray crystal structure analysis of silvaticusin A (1) can be found in in the Supplementary Material (Part 4).
3.6 The cytotoxicity assay
The cytotoxicity assay has been described previously [23].
Data availability
The data that support the findings of this study are openly available in the Science Data Bank at https://doi.org/https://doi.org/10.57760/sciencedb.09413.
References
Sun HD, Huang SX, Han QB. Diterpenoids from Isodon species and their biological activities. Nat Prod Rep. 2006;23(5):673–98.
Liu M, Wang WG, Sun HD, Pu JX. Diterpenoids from Isodon species: an update. Nat Prod Rep. 2017;34(9):1090–140.
Li MX, Yan BC, Zhou M, Li XR, Li XN, He SJ, et al. Cyclobutane-containing meroditerpenoids, (+)-isoscopariusins B and C: structure elucidation and biomimetic synthesis. Org Lett. 2023;25(17):2981–5.
Li XR, Yan BC, Hu K, He SJ, Sun HD, Zuo JP, et al. Spiro ent-clerodane dimers: discovery and green approaches for a scalable biomimetic synthesis. Org Lett. 2021;23(15):5647–51.
Li X, Chen L, Hu K, Yan B, Du X, Li X, et al. Discovery and biological evaluation of dispirocyclic and polycyclic ent-clerodane dimers from Isodon scoparius as novel inhibitors of Toll-like receptor signaling. Org Chem Front. 2022;9(15):4023–33.
Gou LL, Yue GG, Lee JK, Puno PT, Lau CB. Natural product eriocalyxin B suppressed triple negative breast cancer metastasis both in vitro and in vivo. Biochem Pharmacol. 2023;210: 115491.
Cheng P, Lin Y, Xu G. New diterpenoids of Rabdosia macrocalyx: the structure of macrocalin A and macrocalin B. Acta Pharmacol Sin. 1984;19(8):593–8.
Wang JN, Zhang ZR, Che Y, Yuan ZY, Lu ZL, Li Y, et al. Acetyl-macrocalin B, an ent-kaurane diterpenoid, initiates apoptosis through the ROS-p38-caspase 9-dependent pathway and induces G2/M phase arrest via the Chk1/2-Cdc25C-Cdc2/cyclin B axis in non-small cell lung cancer. Cancer Biol Ther. 2018;19(7):609–21.
Wang JN, Che Y, Yuan ZY, Lu ZL, Li Y, Zhang ZR, et al. Acetyl-macrocalin B suppresses tumor growth in esophageal squamous cell carcinoma and exhibits synergistic anti-cancer effects with the Chk1/2 inhibitor AZD7762. Toxicol Appl Pharmacol. 2019;365:71–83.
Shen XY, Isogai A, Furihata K, Sun HD, Suzuki A. Maoecrystal M: a naturally occurring symmetric ent-kaurane dimer from Rabdosia eriocalyx. Phytochemistry. 1994;35(3):725–9.
Yang LB, Yang J, Li LM, Lei C, Zhao Y, Huang SX, et al. Symmetric and asymmetric ent-kaurane dimers isolated from Isodon japonicus. Tetrahedron Lett. 2008;49(22):3574–7.
Yang JH, Wang WG, Du X, He F, Zhang HB, Li XN, et al. Heterodimeric ent-kauranoids from Isodon tenuifolius. J Nat Prod. 2014;77(11):2444–53.
Yang PY, Jia Q, Song SJ, Huang XX. [2+2]-Cycloaddition-derived cyclobutane natural products: structural diversity, sources, bioactivities, and biomimetic syntheses. Nat Prod Rep. 2023;40(6):1094–129.
Hou SY, Yan BC, Sun HD, Puno PT. Recent advances in the application of [2+2] cycloaddition in the chemical synthesis of cyclobutane-containing natural products. Nat Prod Bioprospect. 2024;14(1):37.
Mori S, Shudo K, Ageta T, Koizumi T, Okamoto T. Studies on the constituents of Isodon trichocarpus KUDO. I. Isolation of the constituents and the structures of isodonol, enmedol, and enmenol. Chem Pharm Bull. 1970;18(5):871–83.
Zhao QZ, Chao JH, Wang HQ, Sun HD, Minami Y. Two new diterpenoids from Rabdosia amethystoides. Acta Bot Yunnan. 1983;5:305–9.
Fujita E, Fujita T, Shibuya M. Terpenoids. VII. The structure and absolute configuration of nodosin, a new diterpenoid from Isodon species. Chem Pharm Bull. 1968;16(3):509–15.
Kubo I, Kamikawa T, Kubota T. Studies on constituents of Isodon japonicus Hara: the structures and absolute stereochemistry of isodonal, trichodonin and epinodosin. Tetrahedron. 1974;30(5):615–22.
Fujita E, Fujita T, Shibuya M. Terpenoids. VI. Isolation of enmein and its 3-acetate from Isodon japonicus Hara. Yakugaku Zasshi. 1967;87(9):1076–8.
Grimblat N, Zanardi MM, Sarotti AM. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J Org Chem. 2015;80(24):12526–34.
Node M, Sai M, Fuji K, Fujita E, Takeda S, Unemi N. Antitumor activity of diterpenoids, trichorabdals A, B, and C, and the related compounds: synergism of two active sites. Chem Pharm Bull. 1983;31(4):1433–6.
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–66.
Tan Q, Hu K, Li XN, Yang XZ, Sun HD, Puno PT. Cytotoxic C-20 non-oxygenated ent-kaurane diterpenoids from Isodon wardii. Bioorg Chem. 2023;135: 106512.
Acknowledgements
We thank Service Center for Bioactivity Screening, Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany for bioactivity screening.
Funding
This work was financially supported by the National Science Fund for Distinguished Young Scholars (82325047), Second Tibetan Plateau Scientific Expedition and Research (STEP) program (2019QZKK0502), NSFC-Joint Foundation of Yunnan Province (U2002221), and Youth Innovation Promotion Association CAS (2023409).
Author information
Authors and Affiliations
Contributions
PTP conceived and designed the research; QXH, KH carried out the experiment and wrote the manuscript; PTP, HPH, HDS and KH supervised the whole study and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Pema-Tenzin Puno is the journal's editor but was not involved in the peer review or decisionmaking process in this article. The other authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13659_2024_465_MOESM1_ESM.docx
Supplementary Material 1. Supplementary data associated with this article (1H, 13C NMR, DEPT, HSQC, HMBC, 1H − 1H COSY, NOESY, HREIMS, UV, ECD and IR spectra of silvaticusins A–D (1–4); Computational data of silvaticusin D (4)).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hai, QX., Hu, K., Chen, SP. et al. Silvaticusins A–D: ent-kaurane diterpenoids and a cyclobutane-containing ent-kaurane dimer from Isodon silvaticus. Nat. Prod. Bioprospect. 14, 45 (2024). https://doi.org/10.1007/s13659-024-00465-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13659-024-00465-9