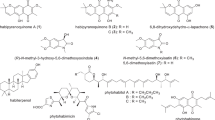
Abstract
The marine holothurian-derived fungal strain KMM 4401 has been identified as Paragliomastix luzulae using 28S rDNA, ITS regions and the partial TEF1 gene sequences. The metabolite profile of the fungal culture was studied by UPLC-MS technique. The strain KMM 4401 is a source of various virescenoside-type isopimarane glycosides suggested as chemotaxonomic feature for this fungal species. Also Px. luzulae KMM 4401 was proposed as possible source of new bioactive secondary metabolites especially antimicrobials. Moreover, the co-cultures of Px. luzulae KMM 4401 with another marine fungus Penicillium hispanicum KMM 4689 inoculated simultaneously or after two weeks were investigated by same way. It was shown, that P. hispanicum KMM 4689 suppressed the production of most of Px. luzulae KMM 4401 metabolites. On the other hand, the co-cultivation of P. hispanicum KMM 4689 and Px. luzulae KMM 4401 resulted in increasing of production of main deoxyisoaustamide alkaloids of P. hispanicum KMM 4689 on 50–190%.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The marine fungal strain KMM 4401 was isolated from the Far Eastern holothurian Eupentacta fraudatrix in 1995 and was identified as Acremonium striatisporum using morphological features [1]. Nearly thirty various diterpene glycosides virescenosides with cytotoxic activities have been isolated from this strain [2,3,4,5]. The first such diterpene glycosides, virescenosides A–H, were isolated from the terrestrial fungus Acremonium luzulae (preferred name Paragliomastix luzulae (Fuckel) L.W. Hou, L. Cai & Crous, 2023) previously incorrectly identified as Oospora virescens (Link) Wallr [6]. It should be noted that diterpene glycosides of this class were found only in these two fungal strains [7].
It has also been reported that A. luzulae can produce cyclosporin C [8]. Currently, undecapeptide cyclosporins A-Z have been isolated from various fungi [9] and were used in many studies due to their antifungal and immunosuppressive activities [10]. Cyclosporine A, known as a drug, is of the greatest interest among them, and numerous studies have been undertaken on the production of cyclosporine A under various cultivation conditions of producing fungi [11]. The authors reported the influence of stress on cyclosporin A production, but co-cultivation has not been described in this sense. Nevertheless, the high antifungal and immunosuppressive activities of cyclosporins can be "beneficial" to the fungus in its interaction with another fungus, and in that case, co-cultivation can stimulate the biosynthesis of cyclosporins or similar compounds. Indeed, fungus-fungus co-cultivation is one of the most relevant approaches for obtaining biologically active compounds [12], and many scientific groups, including ours, are working in this field [13, 14].
Acremonium is a highly polyphyletic genus and recently acremonium-like species have been revised to 63 genera and 14 families in Cephalothecales, Glomerellales, and Hypocreales, mainly in the families Bionectriaceae, Plectosphaerellaceae, and Sarocladiaceae and five new hypocrealean families, namely Chrysonectriaceae, Neoacremoniaceae, Nothoacremoniaceae, Pseudoniessliaceae, and Valsonectriaceae [15]. Based on multi-locus phylogenetic analysis, the new genus Paragliomastix in Bionectriaceae has been proposed to include Px. chiangraiensis (basionym Acremonium chiangraiense), Px. luzulae (basionym Torula luzulae), Px. znieffensis, and a novel species Px. rosea [15]. Therefore, we carried out an identification of the strain KMM 4401 using three molecular markers: 28S rRNA (large subunit ribosomal RNA), ITS and translation elongation factor EF-1 alpha (TEF1).
To determine whether strain KMM 4401 could potentially produce compounds other than those already isolated, its metabolite profile was investigated using UPLC-MS. In addition, the strain KMM 4401 was cultivated with the marine fungus Penicillium hispanicum KMM 4689 at two different inoculation times (simultaneously or sequentially after 14 days) to determine whether and how inoculation time affects the production of secondary metabolites. The marine strain P. hispanicum KMM 4689 was found to be a source of structurally interesting desoxyisoaustamide-related alkaloids [16] with promising bioactivity [17], and several studies on its fermentation have been carried out [18].
Thus, the aim of this study was to identify the marine fungal strain KMM 4401 and investigate its metabolite profile as is and after co-cultivation with P. hispanicum KMM 4689 using two different times for inoculation.
2 Results
2.1 Identification of Paragliomastix luzulae КMM 4401
In this paper, to clarify the taxonomic position of the strain KMM 4401, we sequenced the molecular markers, such as 28S rDNA, ITS regions and the partial TEF1 gene sequence. Approximately 950 bp fragment of the partial 28S rDNA region, about 1600 bp fragment of the ITS region, and about 1000 bp fragment of the partial TEF1 gene sequence were successfully amplified. BLAST search showed that these sequences were 99 to 100% identical with those of the non-ex-type strains Px. luzulae CBS 494.67, CBS 495.67, and CBS 935.69. Phylogenetic ML tree of the concatenated 28S-ITS-TEF1 gene sequences clearly showed that the strain KMM 4401 clusters with the strains Px. luzulae (Fig. 1).
2.2 Metabolite profile of Paragliomastix luzulae КMM 4401
The UPLC MS chromatogram of extract of Px. luzulae (Pl) КMM 4401 culture is presented in Fig. 2.
In total, 27 compounds were annotated using the in-house database or MetFrag service with PubChem database (Fig. 3, Table S1). The detailed characteristics of the identified compounds are presented in the Supplementary Materials (Table S1).
The peak #21 detected at 6.2 min at m/z 659.3271 corresponded to the molecular formula C32H50O14, the same as virescenoside R2, an isopimarane glycoside with a disaccharide moiety isolated early from Px. luzulae KMM 4401 [4]. The compound can be suggested based on an exact mass value. The peak #25 found at 9.2 min at m/z 479.2653 corresponded to either virescenosides T or U, as also confirmed by fragmentation under CID conditions. These two compounds have nearly identical structures, differing only in the position of the double bond in the aglycone. Moreover, four other peaks (#22, 24, 23 and 26) with similar exact mass (m/z 479.2618, 479.2653, 479.2618, and 479.2653) were detected at 6.4, 8.4, 9.1, and 9.6 min, respectively. All of them, have characteristic ion at m/z 317.2103 and 299.2005, arising from losses of altrose by in-source dissociation. Virescenosides T and U were earlier isolated from the KMM 4401 strain [19], and three additional peaks very likely corresponds to previously undescribed virescenosides, which are isomeric to virescenosides T and U.
The peak #9 found at 7.4 min at m/z 497.2737 corresponded to the molecular formula C26H40O9, which can be associated with isomeric virescenosides M and V that were isolated from the KMM 4401 strain [20, 21]. It was also suggested based on MS/MS fragmentation. In addition, three other peaks (#7, 8 and 10) with similar exact mass were detected at 6.8, 7.2 and 7.7 min, respectively. All these peaks, besides the molecular ion peak, have characteristic aglycone peaks at m/z 317.2103 and 335.2217, formed by in-source dissociation. Two of these peaks obviously correspond to unknown isomers of virescenosides M and V.
The peaks #11, 12, and 13 found at 6.9, 7.3, and 7.5 min at m/z 499.2909 corresponded to the molecular formula C26H42O9, which can be associated with virescenosides N [20], W and X [21] that have isomeric aglycones and were earlier described as metabolites of the KMM 4401 strain. The peak #14 found at 8.7 min at m/z 481.2803 corresponded to the molecular formula C26H40O8, which can be associated with isomeric virescenosides P [22] and S [19] that were earlier obtained from this fungal strain. Moreover, four other peaks (#15, 16, 17 and 18) were detected at 8.9, 9.4, 10.0, and 11.0 min, respectively, and have characteristic aglycone peaks at m/z 301.2159 and 319.2260, formed by in-source dissociation. Two of these peaks obviously correspond to unknown isomers of virescenosides N, W, and X [20, 21].
The peaks #19 and 20 detected at 9.6 min and 10.5 min at m/z 645.3461 and 645.3501 corresponded to the molecular formula C32H52O13, which can be associated with the isomeric compounds virescenoside R and R3. Compounds were identified based on an exact mass values and fragmentation patterns. Earlier these compounds were isolated from the KMM 4401 strain [4, 19].
The peaks #1 and 2 detected at 10.6 min and 11.5 min at m/z 483.2961 corresponded to the molecular formula C26H42O8, the same as virescenosides A [23] and O [22], respectively. Compounds were identified based on comparison of exact mass values, fragmentation patterns, and RT with whose of authentic standards.
The peak #3 detected at 12.7 min with m/z 467.2998 corresponded to the molecular formula C26H42O7, the same as virescenoside B [23]. The compound was identified based on comparison an exact mass value, MS/MS fragmentation, and RT with those of virescenoside B.
The peaks #4 and 5 found at 12.4 at m/z 467.2981 and 13.3 min at m/z 467.3015 corresponded to the molecular formula C26H42O7, which can be associated with virescenoside Q, known metabolite of the KMM 4401 strain [22], and another unknown isomeric virescenoside. This was suggested based on characteristic aglycone peaks at m/z 301.2159 and 319.2260, formed by in-source dissociation.
The peak #6 detected at 15.6 min at m/z 303.2325 corresponded to the molecular formula C20H30O2, the same as aglycone of virescenoside C [24]. The compound was identified as virescenoside C based on corresponding MS and RT data from in-house database.
The peak #27 detected at 16.2 at m/z 1176.7974 corresponded to the molecular formula C59H105N11O13, the same as 6-[(3R,4S)-3-hydroxy-N-methyl-5-oxo-L-leucine]cyclosporin A and 9-(N-Methyl-L-serine)cyclosporin A. Compound was annotated based on an exact mass value and the MetFrag service.
The peak #50 detected at 20.4 min at m/z 429.3350 corresponded to the molecular formula C28H44O3, the same as ergosterol peroxide, a usual derivative of main triterpenoid [25]. Compound was identified based on an exact mass value and RT with an in-house database.
Earlier cyclosporin A derivative was synthesized by ozonolysis of cyclosporin methyl vinyl ketone followed by reductive workup and its immunosuppressive properties was patented [26, 27]. Cyclosporin A derivative was reported in the patent as a synthetic compound [28]. So, we proposed that Px. luzulae КMM 4401 may be natural source of these synthetic derivatives of cyclosporin A and this strain should be promising for the isolation of this and others cyclosporin A derivatives for future investigations.
2.3 Metabolite profile of Paragliomastix luzulae and Penicillium hispanicum co-culture with simultaneously inoculation
For this study, the culture of Px. luzulae KMM 4689 were seeded in flasks and P. hispanicum KMM 4689 was inoculated in these flasks immediately. After that the co-culture grown for 3 weeks and then was extracted for following UPLC MS analysis.
Earlier P. hispanicum KMM 4689 axenic culture (Ph) metabolite profile was reported [18] and in this work it was confirmed by UPLC MS technique. 25 compounds were detected in Ph extracts (Supplementary Materials, Table S1).
The UPLC-MS chromatogram of the extract of Px. luzulae KMM 4401 and P. hispanicum KMM 4689 simultaneously inoculated co-culture (PlPh1) in comparison with UPLC-MS chromatogram of Ph are presented in Fig. 4.
A total of 38 compounds were successfully identified using the in-house database and GNPS (Figs. 3 and 5, Table S1). The detailed characteristics of the identified compounds are presented in the Supplementary Materials (Table S1). 17 from these peaks were corresponded to metabolites of Px. luzulae KMM 4401: virescenosides A (#1), B (#3), O (#2), Q/its isomer (#4 and #5), S/P/their isomers (#15–18), R/R3 (#19 and #20), and T/U/their isomer (#24–26), aglycone of virescenoside C (#6), as well as one from the virescenosides M/V/their isomers (#7), and cyclosporin A derivative (#27).
The secondary metabolites of Penicillium hispanicum KMM 4689 [18] detected in the co-cultures
In addition, the compounds previously reported from axenic culture of P. hispanicum KMM 4689 [18] were detected: 3β-hydroxydeoxyisoaustamide (#28), 3,4-dimethoxycinnamic acid (#29), ( +)-deoxyisoaustamide (#38), 3β-hydroxydeoxyisoaustamide (#28), austamide (#32), brevianamide F (#30), 7-hydroxy-3-(2-hydroxypropyl)-5-methylisochromen-1-one (#31), 16β,17α-dihydroxy-deoxydihydroisoaustamide (#33), 16,17-dihydroxydeoxydihydroisoaustamide (#34), 16α,17α-dihydroxy-deoxydihydroisoaustamide (#35), 16α-hydroxy-17β-methoxy-deoxydihydroisoaustamide/16β-hydroxy-17α-methoxy-deoxydihydroisoaustamide/16α-hydroxy-17α-methoxy-deoxydihydroisoaustamide (#36), deoxydihydroisoaustamide (#37), endocrocin (#39), citreorosein (#40), desoxybrevianamide E (#41), 2-chlorocitreorosein (#42), deoxy-14,15-dehydroisoaustamide (#43), emodine(#44), nephrolaevigatin D (#46), nephrolaevigatin C (#47), nephrolaevigatin A (#48), and nephrolaevigatin B (#49).
2.4 Metabolite profile of time delay co-culture of Paragliomastix luzulae and Penicillium hispanicum
Another variant of co-culture was obtained when the fungus Paragliomastix luzulae KMM 4401 was inoculated in the flasks and Penicillium hispanicum KMM 4689 culture was added in these flasks after 14 days. Then this co-culture was fermented for three weeks and then extracted for following UPLC-MS analysis.
The UPLC-MS chromatogram of the extract of Paragliomastix luzulae KMM 4401 and Penicillium hispanicum KMM 4689 co-culture with time delay inoculation (PlPh2) in comparison with UPLC-MS chromatogram of Ph are presented in Fig. 6.
In total, 45 compounds were successfully identified or annotated using the in-house database and GNPS (Figs. 3 and 5, Table S1). These are 20 metabolites of Px. luzulae KMM 4401 including virescenosides A (#1), O (#2), B (#3), Q and its isomer (#4 and #5), virescenoside C aglycone (#6), virescenosides M/V/their isomers (#7–9), virescenoside N or W or X (#11 and #13), virescenoside S/P/their isomers (#14–18), virescenoside R/R3 (#19 and #20), virescenoside T or U or their isomer (#25) and cyclosporin A derivative (#27).
Moreover, 25 compounds earlier detected in axenic culture of P. hispanicum KMM 4689 as 3β-hydroxydeoxyisoaustamide (#28), 3,4-dimethoxycinnamic acid (#29), brevianamide F (#30), 7-hydroxy-3-(2-hydroxypropyl)-5-methylisochromen-1-one (#31), austamide (#32), 16β,17α-dihydroxy-deoxydihydroisoaustamide (#33), 16,17-dihydroxy-deoxydihydroisoaustamide (#34), 16α,17α-dihydroxy-deoxydihydroisoaustamide (#35), 16α-hydroxy-17β-methoxy-deoxydihydroisoaustamide/16β-hydroxy-17α-methoxy-deoxydihydroisoaustamide/16α-hydroxy-17α-methoxy-deoxydihydroisoaustamide (#36), deoxydihydroisoaustamide (#37), ( +)-deoxyisoaustamide (#38), endocrocin (#39), citreorosein (#40), desoxybrevianamide E (#41), 2-chlorocitreorosein (#42), deoxy-14,15-dehydroisoaustamide (#43), emodine (#44), secalonic acid D (#45), and nephrolaevigatins D (#46), C (#47), A (#48), and B (#49), as well as ergosterol peroxide (#50).
2.5 The comparative analysis of metabolite profiles of fungal cultures
The relative content of the announced compounds calculated as a ratio of the peak area to the total area of these peaks in the UPLC-MS chromatogram of Pl, Ph, PlPh1, and PlPh2 extracts was visualized in the heatmap (Fig. 7).
It was found that cyclosporin A derivative (#27) is the main component of Pl extract, but its amount dramatically decreased in both PlPh1 and PlPh2.
( +)-Deoxyisoaustamide (#38) is predominant not only in Ph extract, but in both PlPh1 and PlPh2. Moreover, the content of this alkaloid in PlPh1 extract increased by ~ 190%, and in PlPh2 extract by ~ 60% compared to the monoculture of P. hispanicum KMM 4689. 3β-Hydroxydeoxyisoaustamide (#28) and deoxydihydroisoaustamide (#37) are the second in content in both PlPh1 and PlPh2 extracts with an increase in concentration of ~ 170% and ~ 50% (for 3β-hydroxydeoxyisoaustamide, #28), respectively, and 68% and 12% (for deoxydihydroisoaustamide, #37). In addition, peak #46, corresponding to neprolaevigatin D, was observed only in both co-cultures, and peak #45, corresponding to secalonic acid D, was detected only in PlPh2 co-culture. At the same time, both compounds were previously described as metabolites of the axenic culture of P. hispanicum KMM 4689 [18].
The analysis of UPLC-MS data was also carried out by the principal component analysis (PCA) and both PCA plot and dendrogram are presented in Fig. 8. The PCA model determined that two principal components (PCs) were optimal for describing approximately 80% of the variation in the samples. The first PC accounted for roughly 55% of the variance, while the second PC accounted for about 24% of the variation (Fig. 8a).
The PlPh1 extract has minimal differences from the Ph extract in both components. At the same time, the PlPh2 is even more similar to the Ph in terms of the PC1 component but differs as much as possible from Ph in PC2. Both PlPh1 and PlPh2 differ significantly from the Pl extract in the PC1 component.
The dendrogram confirms the main conclusions of the PCA plot, showing the maximum similarity of PlPh1 and Ph extracts, as well as placing PlPh2 in the same cluster while Pl extract is in another cluster.
2.6 Bioactivity of fungal extracts
The effect of Pl, Ph, PlPh1 and PlPh2 extracts on the urease activity and the growth of Gram-positive bacteria Staphylococcus aureus, Gram-negative bacteria Escherichia coli and yeast-like fungus Candida albicans test strains is presented in Table 1.
The extract of Pl culture inhibited the growth of S. aureus, E. coli and C. albicans by 53.7%, 56.4%, and 20.4%, respectively. At same time, the extract of Ph culture inhibited the growth of S. aureus and E. coli by 29.1% and 34.2%, respectively, and was inactive against C. albicans. The extract of PlPh1 did not show any activity in this test while the extract of PlPh2 inhibited the growth of S. aureus and E. coli by 18.0% and 34.2%, respectively.
The influence of these extracts on human hepatocarcinoma HepG2 and normal rat cardiomyocytes H9c2 cells are presented in Fig. 9.
The viability of HepG2 was decrease by 17–30% when the extracts were used at a 100 µg/mL (Fig. 9a). The extracts Pl, Ph, and PlPh1 at a concentration of 10 µg/mL were nontoxic for HepG2 while PlPh2 caused the decrease of HepG2 viability by near 30%.
The toxic effect of the extracts on H9c2 cell viability was more significant (Fig. 9b). The extracts Pl, Ph, PlPh1, and PlPh2 used at a 100 µg/mL decreased the viability of H9c2 by 66.7%, 45.9%, 52.3%, and 42.8%, respectively. After dilution of the extracts by 10 times, all these decreased the viability of H9c2 by 8.9–18.2%.
DPPH radical scavenging activity of all extracts at a concentration of 100 µg/mL was measured. The extracts Pl, Ph, PlPh1, and PlPh2 scavenged 48.5%, 16.0%, 34.2%, and 49.7% of DPPH radicals.
3 Discussion
Thus, fungal strain KMM4401 identified earlier as A. striatisporum only by morphologic features, has now been re-identified as Px. luzulae using molecular genetic approach. As noted in the Introduction, Acremonium is a taxonomically difficult and highly polyphyletic group of ascomycetes. Combined the phylogenetic trees analysis with morphological characteristics, host and ecological analyses, acremonium-like species were recently combined to 63 genera, and 14 families including five new [15]. According to this new taxonomic system, Paragliomastix luzulae is actual name for synonyms Acremonium luzulae, Sagrahamala luzulae, and Gliomastix luzulae [29].
The detail investigation of metabolite profile of marine fungus Px. luzulae KMM 4401 isolated from holothurian was carried out by UPLC-MS. This made it clear that the secondary metabolites of this strain are more diverse than was understood after a series of publications on isolation and structure determination of its low molecular weight compounds. To date, it is known about 38 isopimarane diterpene glycosides, virescenosides, previously isolated from the strain KMM 4401, but the new data suggest that at least nine other related compounds can be still isolated. It should be note that totally only 118 diterpene glycosides were isolated from various fungi and only 59 from them contain isopimarane aglycones [30]. So, the Px. luzulae KMM 4401 strain produces the third part of all known fungal diterpene glycosides, and our observations made this fungus very interesting for future investigation. The re-identification of the KMM 4401 strain as Px. luzulae and the fact that virescenosides were isolated only from fungi of this species suggests that isopimarane glycosides of this type are a chemotaxonomic marker for these fungi.
Moreover, UPLC-MS data showed that this marine fungus may produce cyclosporin A related peptides which earlier were not reported for this strain. The bioactivity of Px. luzulae KMM 4401 extract further confirms the promise of its further study, since it has been established its significant antimicrobial activity, which was not reported for earlier isolated compounds.
Co-cultivation of fungal strains is a common way to influence the production of their secondary metabolites. In this case, we chose the P. hispanicum KMM 4689 as the second strain for co-cultivation with Px. luzulae KMM 4401.
Earlier it was showed that P. hispanicum KMM 4689 reacted to changes in the composition of the environment by changing the production of secondary metabolites [18], and in a co-culture with marine isolate of Aspergillus fumigatus it produced new metabolites [31]. In present work, P. hispanicum KMM 4689 has some ability to suppress the production of the secondary metabolites of Px. luzulae KMM 4401 that was observed both in UPLC-MS data and antimicrobial activity of extracts. Despite some increase of cytotoxicity of co-cultures extracts toward human hepatocarcinoma HepG2 cells were detected, it does not appear that any new low molecular weight compounds announced in significant quantities in investigated fungal co-cultures. ( +)-Deoxyisoaustamide, 3β-hydroxydeoxyisoaustamide and deoxydihydroisoaustamide from P. hispanicum KMM 4689 were main components in P. hispanicum KMM 4689 and Px. luzulae KMM 4401 co-cultures extracts, but it has not previously been established any their biological activity which can explain the suppress of Px. luzulae KMM 4401 in studied co-cultures. But it is likely that they play some role in this, and it may be aim for future investigation.
Another implication of this work is that the timing of inoculation in co-cultivation of fungi in lab matters. It was observed that P. hispanicum KMM 4689 suppressed the production of secondary metabolites of Px. luzulae KMM 4401 during co-cultivation of these fungi with simultaneous inoculation. At the same time, the production of some P. hispanicum alkaloids increased significantly. When P. hispanicum KMM 4689 was inoculated two weeks later, the production of metabolites of Px. luzulae KMM 4401 increased, but the production of deoxyisoaustamide alkaloids was less than in case of simultaneous inoculation. Deoxyisoaustamide alkaloids have promising biological activities [32, 33] and co-cultivation of P. hispanicum KMM 4689 and Px. luzulae KMM 4401 with simultaneous inoculation can be one of the ways to obtain them in large quantities for future investigations of its bioactivities and semi-synthesis of unique minor derivatives.
4 Conclusions
The marine holothurian-derived fungal strain KMM 4401 was identified as Px. luzulae using 28S rDNA, ITS regions and the partial TEF1 gene sequence. This fungus was confirmed as a source of various glycosides as well as possible source of new bioactive secondary metabolites especially antimicrobials. The co-cultivation of Px. luzulae KMM 4401 with another marine fungus P. hispanicum KMM 4689 resulted in the reducing of the secondary metabolites’ production by Px. luzulae fungus. The co-cultivation of P. hispanicum KMM 4689 and Px. luzulae KMM 4401 with simultaneous inoculation can be considered as a promising way to obtain the new metabolites in large quantities for future investigations.
5 Materials and methods
5.1 General
Microscopic examination and photography of fungal cultures were performed with an Olympus CX41 microscope fitted with an Olympus SC30 digital camera. Detailed examination of ornamentation of the fungal conidia was performed using scanning electron microscopy (SEM) EVO 40.
Low-pressure liquid column chromatography was performed using a Gel ODS-A (12 nm, S—75 um, YMC Co., Ishikawa, Japan). Plates precoated with Si gel (5–17 μm, 4.5 cm × 6.0 cm, Imid Ltd., Krasnodar, Russia) were used for thin-layer chromatography.
5.2 Fungal strains
The fungal strain KMM 4401 was isolated from superficial mycobiota of the sea cucumber Eupentacta fraudatrix collected in the Kitovoye Rebro Bay (the Sea of Japan) and identified based on morphological features as Acremonium striatisporum [20]. The fungal strain KMM 4689 was isolated from unidentified soft coral collected near Con Co Island (the South China Sea, Vietnam) and identified based on molecular features as Penicillium hispanicum [18].
All used fungal strains are stored in the Collection of Marine Microorganisms (PIBOC FEB RAS, Vladivostok, Russia). The strain Penicillium hispanicum KMM 4689 also stored in the Collection of Nhatrang Institute of Technology Research and Applications under the code VO49-30.5.
5.3 DNA extraction and amplification
Genomic DNA was isolated from fungal mycelia (mycelium) grown on MEA (malt extract agar) at 25 °C for seven days, using the MagJET Plant Genomic DNA Kit (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s protocol. PCR was conducted using GoTaq Flexi DNA Polymerase (Promega, Madison, WI, USA). The 28S rDNA region was amplified using the standard primer pair LROR and LR5 [34]. The reaction profile was 95 °C for 300 s, 35 cycles of 95 °C for 30 s, 55 °C for 45 s, and 72 °C for 120 s, and finally 72 °C for 300 s. For amplification of the internal transcribed spacer region (ITS) were used the primer pair ITSpr1 (5′-GCGTTGATATACGTCCCTGCC-3′) [35] and ITSpr7 (previously named as D3B*-R) (5′-ACTTCGGAGGGAACCAGCTAC-3′) [36]. The reaction profile was 95 °C for 300 s, 35 cycles of 95 °C for 20 s, 55 °C for 20 s, and 72 °C for 120 s, and finally 72 °C for 300 s. For amplification of the TEF1 gene the standard primer pair EF1-983F and EF1-2218R was used [37]. The reaction profile was 95 °C for 300 s, 35 cycles of 95 °C for 30 s, 50 °C for 45 s, and 72 °C for 90 s, and finally 72 °C for 300 s. The amplified 28S rDNA, ITS and TEF1 fragments were purified and sequenced as described in [38]. Gene sequences were deposited in GenBank under accession numbers OR650021 for 28S, OR650019 for ITS and OR672062 for TEF1 (Table 2).
5.4 Phylogenetic analysis
The 28S rDNA, ITS and TEF1 nucleotide sequences of the fungal strain KMM 4401 and members of the genus Paragliomastix of the family Bionectriaceae were aligned by MEGA X software version 11.0.9 [39] using Clustal W algorithm. The ex-type homologs and non-ex-type homologs were searched in the GenBank database (http://ncbi.nlm.nih.gov) using the BLASTN algorithm (http://www.ncbi.nlm.nih.gov/BLAST, accessed on 02 October 2023). The phylogenetic analysis was conduct using MEGA X software [39]. The 28S rDNA, ITS regions and partial TEF1 gene sequences were concatenated into one alignment. Phylogenetic tree was constructed according to the Maximum Likelihood (ML) algorithm based on the Tamura 1992 model [40]. The tree topology was evaluated by 1000 bootstrap replicates. The Gliomastix musae CBS 617.94T was used in the phylogenetic analysis as outgroup (Table 2).
5.5 Cultivation of fungi
Before co-cultivation, fungal strains Px. luzulae KMM 4401 и P. hispanicum KMM 4689 were grown in test tubes on slanted wort agar in sea water for 14 days at 22 °C. Subsequently, the resulting fungal cultures were used for cultivation on rice medium. Sowing for joint growth was carried out in two variants: in the first case, inocula of 2-week-old fungal strains were introduced into flasks with rice medium simultaneously, in the second case—with a difference of two weeks, considering the different growth rates of the studied crops. In the second case, the inoculum of P. luzulae was first added to the medium and cultivated at 22 °C for two weeks. After 14 days, an inoculum of P. hispanicum was added to the same flask. Co-cultivation of the strains was carried out at room temperature for 21 days.
5.6 Extraction of fungal cultures
Each fungal culture with medium was extracted with EtOAc (100 mL) and then evaporated in vacuo to yield a crude extract (Table 3). Then each extract was dissolved in methanol and passed through column with C18-SiO2 (YMC Gel ODS-A). The masses of purified extracts are presented in Table 3.
5.6.1 UPLC-MS analysis of fungal extracts
UPLC-MS analysis was performed using a Bruker Elute UPLC chromatograph (Bruker Daltonics, Bremen, Germany) connected to a Bruker Impact II Q-TOF mass spectrometer (Bruker Daltonics, Bremen, Germany). An InfinityLab Poroshell 120 SB-C18 column (2.1 × 150 mm, 2.7 μm, Agilent Technologies, Santa Clara, CA, USA) was used for chromatographic separation. The detailed description of chromatographic separation and mass spectrometric detection were reported earlier [18].
5.6.2 UPLC-Q-TOF data analysis
UPLC-Q-TOF data were converted from Bruker “.d” formatting to “.mzXML” using MSConvert 3.0 (part of ProteoWizard 3.0 package, Palo Alto, CA, USA) [41], and further processing was performed using MZMine (version 2.53) [42] as described previously [18].
In addition, the identification of some metabolites was performed by comparing of experimental MS/MS spectra with compounds from the PubChem database using in-silica fragmentation by MetFrag service [43]. Metabolite dereplication was also carried out with an in-house MS/MS spectral library [18].
5.7 Principal component analysis ((PCA)
PCA analysis, a hierarchical dendrogram, and visualization of the resulting graphs were performed using the “google colab” web resource based on Python 3.8 using Pandas, Seaborn, and Matplotlib libraries. Below is a link to the notepad with the code used in the analysis:https://drive.google.com/drive/folders/1gTZMlPZXXbFH3UBE5FjaTMzETgmqmYjr?usp=sharing
5.8 Bioassays
5.8.1 Urease inhibition assay
The urease inhibitory activity was estimated by determining ammonia production using the indophenol method. Urease from Canavalia ensiformis (final concentration 1U) was used in this test. A reaction mixture consisting of 25 µL enzyme solution and 5 µL of extracts (100.0 µg/mL final concentration) was preincubated at 37 °C for 60 min in 96-well plates. Then, 55 µL of phosphate buffer solution with 100 µM urea was added to each well and incubated at 37 °C for 10 min. Then, 45 µL of phenol reagent (1% w/v phenol and 0.005% w/v sodium nitroprusside) and 70 µL of alkali reagent (0.5% w/v NaOH and 0.1% active chloride NaClO) were added to each well. The pH was maintained at 7.3–7.5 in all assays. DMSO 5% was used as a positive control. The absorbance was measured after 50 min at 630 nm using a MultiskanFS microplate reader (Thermo Scientific Inc., Beverly, MA, USA).
5.8.2 Antimicrobial activity
The Gram-positive bacteria Staphylococcus aureus ATCC 21027, Gram-negative bacteria Escherichia coli VKPM (B-7935) and yeast-like fungi Candida albicans KMM 455 strains were fermented on solid medium Mueller Hinton broth with agar (16.0 g/L) in a Petri dish at 37 °C for 24 h.
The assays were performed in 96-well microplates in appropriate Mueller Hinton broth. The test strains’ suspensions (90 µl, 106 CFU/mL) were added in each well and then 10 µL of the extract diluted at concentrations from 12.5 µg/mL to 100.0 µg/mL using two-fold dilution was added. The concentration of vehicle (DMSO) was less than 1%. The antibiotic gentamicin and the antifungal agent nitrofungin were used as positive controls at 1 mg/mL. Moreover, DMSO (1% in PBS) served as a negative control. The plates were incubated for 18 h at 37 °C, and the OD620 was measured using a Multiskan FS spectrophotometer (Thermo Scientific Inc., Beverly, MA, USA). The inhibition of test strains’ growth was calculated as % = (ODcontrol – ODsample)/ODcontrol*100% [44].
5.8.3 DPPH radical scavenger assay
The methanol solution of DPPH (Sigma-Aldrich, Steinheim, Germany) at a concentration of 7.5 × 10−3 M was used. The dry extracts were dissolved in MeOH at a concentration of 125 µg/mL, and the final concentration in reactive mixture was 100 µg/mL. The optical density of the mixture after 30 min was detected at 520 nm with a microplate reader MultiscanFC (ThermoScientific, USA). The radical scavenging activities of the extracts at 100 µg/mL were calculated as % of the control (MeOH).
5.8.4 Cell culture
The human hepatocarcinoma HepG2 HB-8065 ™cells were purchased from ATCC (Manassas, VA, USA). The rat cardiomyocytes H9c2 cells were kindly provided by Prof. Dr. Gunhild von Amsberg from Martini-Klinik Prostate Cancer Center, University Hospital Hamburg-Eppendorf, Hamburg, Germany. The cells were cultured in DMEM with 10% of fetal bovine serum and 1% of penicillin/streptomycin (BioloT, St. Petersburg, Russia). For experiments HepG2 and H9c2 cells were seeded at concentrations of 5 × 103 cell/well and 3 × 103 cell/well, respectively, and the experiments were started after 24 h.
5.8.5 Cell viability assay
The cells were treated with extracts at a concentration of 10 µg/mL and 100 µg/mL for 24 h, and the viability of cells was measured using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay according to the manufacturer’s instructions (Sigma-Aldrich, St.-Louis, MO, USA). The results are presented as a percentage of control data.
5.8.6 Statistical data evaluation
All bioassays’ data were obtained in three independent replicates, and calculated values are expressed as a mean ± standard error mean (SEM) using SigmaPlot 14.0 (Systat Software Inc., San Jose, CA, USA).
Availability of data and materials
The original data presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
References
Pivkin MV. Filamentous fungi associated with holothurians from the sea of Japan, off the primorye coast of Russia. Biol Bull. 2000;198:101–9.
Marcotullio MC, Rosati O, Curini M. Virescenols: sources, structures and chemistry. Nat Prod Commun. 2008;3:1934578X0800300627.
Afiyatullov SS, Kalinovsky AI, Antonov AS. New virescenosides from the marine-derived fungus Acremonium striatisporum. Nat Prod Commun. 2011;6:1934578X1100600803.
Afiyatullov SS, Kalinovsky AI, Antonov AS, Zhuravleva OI, Khudyakova YV, Aminin DL, et al. Isolation and structures of virescenosides from the marine-derived fungus Acremonium striatisporum. Phytochem Lett. 2016;15:66–71.
Zhuravleva OI, Antonov AS, Oleinikova GK, Khudyakova YV, Popov RS, Denisenko VA, et al. Virescenosides from the holothurian-associated fungus Acremonium striatisporum KMM 4401. Mar Drugs. 2019. https://doi.org/10.3390/md17110616.
Cagnoli-Bellavita N, Ceccherelli P, Ribaldi M, Polonsky J, Baskevitch-Varon Z, Varenne J. Structures of virescenosides D and H, new metabolites of Acremonium luzulae (Fuckel) Gams. J Chem Soc Perkin Trans 1. 1977. https://doi.org/10.1039/p19770000351.
Hussain H, Mamadalieva NZ, Ali I, Elizbit, Green IR, Wang D, et al. Fungal glycosides: structure and biological function. Trends Food Sci Technol. 2021;110:611–51.
Moussaïf M, Jacques P, Schaarwächter P, Budzikiewicz H, Thonart P. Cyclosporin C is the main antifungal compound produced by Acremonium luzulae. Appl Environ Microbiol. 1997;63:1739–43.
Wang X, Lin M, Xu D, Lai D, Zhou L. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides. Molecules. 2017. https://doi.org/10.3390/molecules22122069.
Corbett KM, Ford L, Warren DB, Pouton CW, Chalmers DK. Cyclosporin structure and permeability: from A to Z and beyond. J Med Chem. 2021;64:13131–51.
Falah F, Vasiee A, Ramezani M, Tabatabaee-Yazdi F, Mortazavi SA, Danesh A. Effect of immobilization, mutation, and microbial stresses on increasing production efficiency of “Cyclosporin A.” Biomass Convers Biorefin. 2022. https://doi.org/10.1007/s13399-022-02533-x.
Fuhrman JA. Microbial community structure and its functional implications. Nature. 2009;459:193–9.
Hu HL, van den Brink J, Gruben BS, Wösten HAB, Gu JD. de Vries RP Improved enzyme production by co-cultivation of Aspergillus niger and Aspergillus oryzae and with other fungi. Int Biodeterior Biodegrad. 2011;65:248–52.
Oppong-Danquah E, Budnicka P, Blümel M, Tasdemir D. Design of fungal co-cultivation based on comparative metabolomics and bioactivity for discovery of marine fungal agrochemicals. Mar Drugs. 2020. https://doi.org/10.3390/md18020073.
Hou L, Giraldo A, Groenewald J, Rämä T, Summerbell R, Huang G, et al. Redisposition of acremonium-like fungi in Hypocreales. Stud Mycol. 2023. https://doi.org/10.3114/sim.2023.105.02_supp.
Zhuravleva OI, Antonov AS, Trang VTD, Pivkin MV, Khudyakova YV, Denisenko VA, et al. New deoxyisoaustamide derivatives from the coral-derived fungus Penicillium dimorphosporum KMM 4689. Mar Drugs. 2021;19:553.
Dyshlovoy SA, Zhuravleva OI, Hauschild J, Busenbender T, Pelageev DN, Yurchenko AN, et al. New marine fungal deoxy-14,15-dehydroisoaustamide resensitizes prostate cancer cells to enzalutamide. Mar Drugs. 2023. https://doi.org/10.3390/md21010054.
Nesterenko LE, Popov RS, Zhuravleva OI, Kirichuk NN, Chausova VE, Krasnov KS, et al. A study of the metabolic profiles of Penicillium dimorphosporum KMM 4689 which led to its re-identification as Penicillium hispanicum. Fermentation. 2023. https://doi.org/10.3390/fermentation9040337.
Afiyatullov SS, Kalinovsky AI, Kuznetsova TA, Pivkin MV, Prokofeva NG, Dmitrenok PS, et al. New glycosides of the fungus Acremonium striatisporum isolated from a sea cucumber. J Nat Prod. 2004;67:1047–51.
Afiyatullov SS, Kuznetsova TA, Isakov VV, Pivkin MV, Prokofeva NG, Elyakov GB. New diterpenic altrosides of the fungus Acremonium striatisporum isolated from a sea cucumber. J Nat Prod. 2000;63:848–50.
Afiyatullov SS, Kalinovsky AI, Pivkin MV, Dmitrenok PS, Kuznetsova TA. New diterpene glycosides of the fungus Acremonium striatisporum isolated from a sea cucumber. Nat Prod Res. 2006;20:902–8.
Afiyatullov SS, Kalinovsky AI, Kuznetsova TA, Isakov VV, Pivkin MV, Dmitrenok PS, et al. New diterpene glycosides of the fungus Acremonium striatisporum isolated from a Sea Cucumber. J Nat Prod. 2002;65:641–4.
Cagnoli-Bellavita N, Cecherelli P, Ribaldi M, Polonsky J, Baskevitch Z. Virescenoside A and virescenoside B, new altroside metabolites of Oospora virescens. Gazz Chim Ital. 1969;99:1354–63.
Cagnoli-Bellavita N, Ceccherelli P, Mariani R, Polonsky J, Baskevitch Z. Structure du virescenoside C, nouveau métabolite de Oospora virescens (Link) Wallr. Eur J Biochem. 1970;15:356–9.
Wieland P, Prelog V. Über die Isolierung von Ergosterin, Ergosterin-palmitat und Ergosterin-peroxyd aus dem Mycel von Aspergillus fumigatus, mut. Helvola, Yuill. Helv Chim Acta. 1947;30:1028–30.
Yang Z, Pattamana K, Molino BF, Haydar SN, Cao Y, Bois F, et al. Novel oxidation of cyclosporin a: preparation of cyclosporin methyl vinyl ketone (Cs-MVK). Synlett. 2009;2009:2935–8.
Or YS, Lazarova T, Chen JS-H. Cyclosporins for the treatment of immune disorders. 2006.
Huang Z, Long Z, Su Z, Yang S. Novel cyclosporin derivatives for the treatment and prevention of a viral infection. 07.06.2012, 2017.
MB#323050. https://www.mycobank.org/page/Name%20details%20page/field/Mycobank%20%23/323050 . Accessed 06 Mar 2024.
Hussain H, Mamadalieva NZ, Ali I, Green IR, Wang D, Zou L, et al. Fungal glycosides: structure and biological function. Trends Food Sci Technol. 2021;110:611–51.
Yurchenko AN, Nesterenko LE, Popov RS, Kirichuk NN, Chausova VE, Chingizova EA, et al. The metabolite profiling of Aspergillus fumigatus KMM4631 and its co-cultures with other marine fungi. Metabolites. 2023. https://doi.org/10.3390/metabo13111138.
Zhuravleva OI, Antonov AS, Trang VTD, Pivkin MV, Khudyakova YV, Denisenko VA, et al. New deoxyisoaustamide derivatives from the coral-derived fungus Penicillium dimorphosporum KMM 4689. Mar Drugs. 2021. https://doi.org/10.3390/md19010032.
Dyshlovoy SA, Zhuravleva OI, Hauschild J, Busenbender T, Pelageev DN, Yurchenko AN, et al. New marine fungal deoxy-14, 15-dehydroisoaustamide resensitizes prostate cancer cells to enzalutamide. Mar Drugs. 2023. https://doi.org/10.3390/md21010054.
Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–46.
Scholin CA, Herzog M, Sogin M, Anderson DM. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J Phycol. 1994;30:999–1011.
Fehling J, Green DH, Davidson K, Bolch CJ, Bates SS. Domoic acid production by Pseudo-nitzschia seriata (Bacillariophyceae) in Scottish waters. J Phycol. 2004;40:622–30.
Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–6.
Belousova EB, Zhuravleva OI, Yurchenko EA, Oleynikova GK, Antonov AS, Kirichuk NN, et al. New anti-hypoxic metabolites from co-culture of marine-derived fungi Aspergillus carneus KMM 4638 and Amphichorda sp. KMM 4639. Biomolecules. 2023. https://doi.org/10.3390/biom13050741.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547.
Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+ C-content biases. Mol Biol Evol. 1992;9:678–87.
Chambers MC, MacLean B, Burke R, Amodei D, Ruderman DL, Neumann S, et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 2012;30:918–20.
Pluskal T, Castillo S, Villar-Briones A, Orešič M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010. https://doi.org/10.1186/1471-2105-11-395.
Ruttkies C, Schymanski EL, Wolf S, Hollender J, Newmann S. MetFrag relaunched: incorporating strategies beyond in silico fragmentation. J Cheminform. 2016;8:3.
Campbell J. High-throughput assessment of bacterial growth inhibition by optical density measurements. Curr Protoc Chem Biol. 2010;2:195–208.
Acknowledgements
The study was carried out using the Collective Facilities Center “Collection of Marine Microorganisms PIBOC FEB RAS” and on the equipment of the Collective Facilities Center “The Far Eastern Center for Structural Molecular Research (NMR/MS) PIBOC FEB RAS”.
Funding
This research was funded by a grant from the Ministry of Science and Higher Education of the Russian Federation, 15.BRK.21.0004 (Contract No. 075-15-2021-1052).
Author information
Authors and Affiliations
Contributions
Conceptualization, E.A.Y. and A.N.Y.; methodology, R.S.P., M.P.I., E.A.Y. and A.N.Y.; software, M.P.I., E.A.Y. and A.N.Y.; validation, M.P.I., E.A.Y. and A.N.Y.; formal analysis, N.N.K., V.E.C. and E.A.C.; investigation, S.S.S., L.E.N., R.S.P., N.N.K., V.E.C., E.A.C. and A.R.C.; resources, M.P.I. and A.N.Y; data curation, A.N.Y.; writing—original draft preparation, S.S.S., L.E.N., R.S.P., N.N.K. and V.E.C.; writing—review and editing, M.P.I., E.A.Y. and A.N.Y.; visualization, S.S.S., V.E.C. and E.A.C.; supervision, M.P.I. and A.N.Y.; project administration, M.P.I. and A.N.Y.; funding acquisition, M.P.I. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no existing competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Starnovskaya, S.S., Nesterenko, L.E., Popov, R.S. et al. Metabolite profiles of Paragliomastix luzulae (formerly named as Acremonium striatisporum) KMM 4401 and its co-cultures with Penicillium hispanicum KMM 4689. Nat. Prod. Bioprospect. 14, 38 (2024). https://doi.org/10.1007/s13659-024-00459-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13659-024-00459-7