Abstract
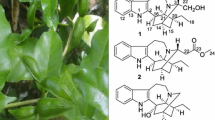
Schisanpropinoic acid (1), a new bergamotane sesquiterpenoid, and schisanpropinin (2), a new tetrahydrofuran lignan with a rare epoxyethane unit, were identified from the stems and leaves of Schisandra propinqua var. propinqua. Their structures were determined based on comprehensive spectroscopic and mass spectrometric analysis. The absolute configuration of 1 was determined by X-ray analysis. Compounds 1 and 2 were tested for their cytotoxic activity against five human tumor cell lines.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Schisandraceae family are a group of economically and medicinally valuable climbing woody vine plants, some of which are used as traditional Chinese medicines in Chinese folk for over 2000 years [1]. Since 1970s, researches on the plants of Schisandraceae family have been a hot topic [2]. In 2003, micrandilactone A, a highly oxygenated, skeleton rearranged, polycyclic nortritepenoid, was reported from S. micrantha by our group [3]. This new finding pushed the studies on the plants of Shisandraceae to a new climax. As a result, last ten years have seen the isolation and identification of large numbers of Schisandra nortriterpenoids (SNTs) from the Schisandraceae family [2, 4].
S. propinqua (Wall.) Baill. var. propinqua is indigenous in Yunnan Province. Previous chemical investigation into this species has led to the identification of a series of new terpenoids and lignans, such as propindilactones E-O [5, 6], lanopropic acid [7], methylisogomisin O [8], and propindilactones T and U [9]. Our further study on this species, which was collected from Weixi county, Yunnan Province, People’s Republic of China to discover new compounds with interesting bioactivity led to the discovery of a new sesquiterpenoid and a new lignan (Fig. 1). This article discribes the isolation, structure elucidation and bioactivities of these two new compounds.
2 Results and Discussion
Compound 1, obtained as colorless crystals (MeOH). Its IR absorptions at 1701 and 1642 cm−1 implied the existence of C=O and C=C groups. The molecular formula was determined as C15H20O3 from the HRESIMS ion at m/z 271.1304 [M + Na]+ (calcd for C15H20O3Na 271.1305) with 6 degrees of unsaturation. The 13C NMR spectrum of 1 displayed only 13 carbon signals. A careful analysis of 1D NMR data (Table 1) and HRESIMS spectrum combined with the HSQC and HMBC spectra (Fig. 2) allowed the assignment of 1 as a sesquiterpenoid with three singlet methyls, three methylenes, two methines, one quaternary carbon, two trisubstituted double bond, one ester carboxyl groups and one carbonyl group. Thus, 1 was a sesquiterpenoid with two rings. The 1H–1H COSY correlations between H-8/H-9/H-10 (Fig. 2) and the HMBC correlations from H-10 (δ H 7.17, 1H, t, J = 7.0 Hz) to C-13 (δ C 13.1) and C-12 (δ C 171.0), and from H-9 (δ H 1.87, 2H, m) to C-6 (δ C 56.6) proved the presence of the side chain. Furthermore, the HMBC correlations from H-1 (δ H 2.34, 1H, br t, J = 6.0 Hz) and H-5 (δ H 2.83, 1H, br t, J = 6.0 Hz) to C-3 (δ C 122.0) and C-14 (δ C 19.1), from H-1 and H-3 (δ H 5.87, 1H, m) to C-15 (δ C 23.4), from H-3 and H-5 to C-4 (δ C 202.5), and from H-9 to C-6 revealing the six-membered ring unit. The 1H-1H COSY correlations of H-1/H-5, H-5/H-7, and H-1/H-7 (Fig. 2) seemed to disclose the existence of cyclopropane unit, while it was confronted with the HMBC correlations from H-7 (δ H 1.92, 1H, d, J = 9.3 Hz; 2.63, 1H, dt, J = 9.3, 6.0 Hz) to C-2 (δ C 170.0), C-4, and C-6. Given a special long-range spin–spin coupling of H-1/H-5 (4 J 1,5 = 6.0 Hz) that subsequently occurred in the cyclobutane unit [10, 11], a cyclobutane unit other than cyclopropane might be existed. This deduction was consistent with all the above-mentioned HMBC correlations. Herein, a structure of cyclobutane adjoined with cyclohexane, along with a side chain unit was elucidated. Compound 1 was a bergamotane-type sesquiterpenoid [12, 13].
Even though the structure of 1 was deduced from extensive spectroscopic analysis, no available correlations in the ROESY spectrum were observed (Fig. 2). Thus, the structure of 1, especially the absolute configuration still needed to be further confirmed. Luckily, a single-crystal X-ray diffraction was performed successfully (Fig. 3). Through structural refinement, the Flack parameter with 0.0(4) and the Hooft parameter at 0.03(17) for 1371 Bijvoet pairs [14, 15], allowed an explicit assignment of the absolute configuration of 1 as 1S, 5S, 6S. Finally, the structure of 1 was determined, and named schisanpropinoic acid.
It is worth noting that 1 was the first sesquiterpenoid with a bergamotane skeleton from Schisandraceae family. In addition, bergamotane sesquiterpenoids were infrequently reported in plants [12, 13, 16–18], and recent discoveries of new bergamotane-type sesquiterpenoids were resulted from fungi resources [19–26].
Compound 2, obtained as a yellowish solid (MeOH). Its IR absorptions at 3424, 1607, 1518 and 1462 cm−1 implied the presence of OH and phenyl groups. Its molecular formula was determined as C20H22O8 by HRESIMS at m/z 389.1237 [M − H]− (calcd for C20H21O8 389.1242), indicating 10 indices of hydrogen deficiency of 2. 13C NMR spectrum (Table 1) displayed only ten carbon signals (six aromatic carbons, one oxygenated sp 3 quaternary carbon, one oxygenated sp 3 methine, one oxygenated methylene, and one methoxy group), suggesting the symmetrical structure of 2. The 1H NMR signals (Table 1) at δ H 7.34 (1H, dd, J = 8.0, 1.8 Hz), 7.54 (1H, d, J = 1.8 Hz), and 7.30 (1H, d, J = 8.0 Hz) indicating the 1,2,4-trisubstituted benzene. This deduction was also confirmed by the 1H–1H COSY (Fig. 4) correlation between H-5 and H-6, as well as the HMBC correlations (Fig. 4) from H-7 (δ H 5.47, 1H, s) to C-1 (δ C 129.9), C-2 (δ C 113.2) and C-6 (δ C 121.6). Taking the lignans from Schisandraceae family into consideration, the above-mentioned data indicated that 2 is identical to the characteristics of a 4H-furan-type lignan with an extra oxygen-containing ring. The HMBC correlations from H-2 and H-6 to C-4, and from methoxyl (δ H 3.72, 3H, s) to C-3 (δ C 148.6) suggested that the methoxy group was located at C-3 rather than C-4. The oxygen-containing ring was deduced to be formed between C-8 and C-8′ by HMBC correlations from H-7 to C-8 (δ C 89.4), from H-9 (δ H 4.54 2H, s) to C-8, and from H-7 to C-9 (δ C 77.0). Thus, the planar structure of 2 was determined. In the ROESY spectrum, both the correlation (Fig. 4) between H-9 and H-7 as well as the confusing correlations of H-2, H-6 and H-9 were observed. Then, a computer-aided 3D molecular model study of two possible relative structure of 2 was conducted. As a result, when H-9 and H-7 were at a same orientation, the internuclear distance between H-9 and H-7 was less than 3.0 Å. On the contrary, the internuclear distance was lager than 3.0 Å. At the same time, the confusing ROESY correlations of H-2, H-6 with H-9 could be explained by the free rotation of the C1–C7 bond. Considering all these factors, the chemical structure of 2 with a relative configuration was determined (Fig. 1). 2 was named schisanpropinin and was a tetrahydrofuran lignan with a rare epoxyethane unit from Schisandraceae family [27].
Compounds 1 and 2 were tested for their cytotoxicity against HL-60, A-549, SMMC-7721, MCF-7, and SW-480 human tumor cell lines by MTS methods using cisplatin and paclitaxel as positive controls. Neither of them showed cytotoxic any activity against all cell lines with IC50 values >40 µM (cisplatin: IC50 = 3.13, 19.05, 13.07, 23.64, and 11.25 µM, respectively, paclitaxel: all <0.008 µM).
3 Experimental
3.1 General
1D and 2D NMR spectra were recorded on Bruker DRX 500 or 600 spectrometers using C5D5N as the internal standard. Chemical shifts (δ) are expressed in ppm relative to the C5D5N signals. HRESIMS was performed on an API QSTAR Pulsar i spectrometer. UV spectra were obtained on a Shimadzu UV-2401PC spectrophotometer. IR spectra were obtained on a Bruker Tensor-27 FT-IR spectrometer using KBr pellets. Optical rotations were measured in MeOH with JASCO P-1020 polarimeters. Column chromatography (CC) was performed with silica gel (100–200 mesh; Qingdao Marine Chemical, Inc., Qingdao, People’s Republic of China), MCI gel (CHP20P, 75–150 μm, Mitsubishi Chemical Corporation, Tokyo, Japan). Semi-preparative HPLC was performed on an Agilent 1200 liquid chromatograph with a Zorbax SB-C18 (9.4 mm × 250 mm) column. Fractions were monitored by thin layer chromatography, spots were visualized by UV light (254 and 365 nm) and by heating silica gel plates sprayed with 10% H2SO4 in EtOH. All solvents used in column chromatography were distilled including petroleum ether (60–90 °C).
3.2 Plant Material
The stems and leaves of S. propinqua var. propinqua were collected in Weixi county, Yunnan Province, People’s Republic of China, in July 2013 and identified by Prof. Xi-Wen Li at Kunming Institute of Botany. A voucher specimen (KIB 2013071801) has been deposited in the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
3.3 Extraction and Isolation
The air-dried and powdered stems and leaves of S. propinqua var. propinqua (21.0 kg) were extracted with 70% aqueous acetone (4 × 40 L, 3 days each) at room temperature. The extract was distilled under reduced pressure to remove acetone, and then partitioned between EtOAc and H2O to afford the EtOAc fraction. EtOAc fraction (690 g) was applied to a silica gel column and eluted with CHCl3/Me2CO (1:0, 9:1 8:2, 7:3, 1:1, 0:1) to give 6 fractions A-F.
Fraction B (9:1, 153 g) was decolorized on MCI gel with 90% MeOH/H2O and a repeated MCI gel CC with MeOH/H2O (60:40, 80:20, 100:0) to obtain B1–B3. Fraction B2 (1.6 g) was separated on RP-18 with MeOH/H2O (40:60, 45:55, 50:50, 55:45, 60:40, 65:35, 70:30, 100:0) to obtain B21–B28. B21 (58 mg) was purified by semi-preparative HPLC to afford 1 (16 mg).
Fraction D (7:3, 99 g) was decolorized on MCI gel with 90% MeOH/H2O to remove pigments and then on RP-18 with MeOH/H2O (30:70, 40:60, 50:50, 60:40, 70:30, 100:0) to afford fractions D1–D6. D2 (8.5 g) was separated by repeated RP-18 with MeOH/H2O (30:70, 35:65, 40:60, 45:55, 50:50, 60:40, 100:0) to afford D21–D25, and D22 (1.1 g) was separated by silica gel CC using light petroleum/acetone and separated out 2 (10 mg).
3.4 The Cytotoxicity Assay
The human tumor cell lines HL-60, SMMC-7721, A-549, MCF-7, and SW-480 were used in the cytotoxic assay. These cell lines were obtained from ATCC (Manassas, VA, USA). Cells were cultured in RMPI-1640 or DMEM medium (Biological Industries, Kibbutz Beit-Haemek, Israel) supplemented with 10% fetal bovine serum (Biological Industries) at 37 °C in a humidified atmosphere with 5% CO2. The cytotoxicity assay was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) (Promega, Madison, WI, USA) assay [28]. Briefly, cells were seeded into each well of a 96-well cell culture plate. After 12 h of incubation at 37 °C, the test compound (40 μM) was added. After incubated for 48 h, cells were subjected to the MTS assay [29, 30]. Compounds with a growth inhibition rate of 50% were further evaluated at concentrations of 0.064, 0.32, 1.6, 8, and 40 μM in triplicate, with cisplatin and paclitaxel (Sigma, St. Louis, MO, USA) as positive controls. The IC50 value of each compound was calculated with Reed and Muench’s method [31].
Schisanpropinoic acid (1): colorless crystals (MeOH). \(\left[ \alpha \right]_{\text{D}}^{20}\) −167.0 (c 0.12, MeOH). UV (MeOH) λ max (log ε) 250 (3.85), 209 (4.18) nm. IR (KBr) v max 2983, 2932, 1701, 1642, 1432, 1381, 1342, 1239, 1207, 1129, 1306, 902 cm−1. ESIMS m/z 271 [M + Na]+, HRESIMS m/z 271.1304 [M + Na]+ (calcd for C15H20O3Na 271.1305). 1H NMR (C5D5N, 500 MHz) and 13C NMR (C5D5N, 125 MHz), see Table 1.
Crystallographic data for 1: C15H20O3, M = 248.31, monoclinic, a = 7.1516(7) Å, b = 23.527(2) Å, c = 7.8731(7) Å, α = 90.00°, β = 91.136(6)°, γ = 90.00°, V = 1324.5(2) Å3, T = 100(2) K, space group P21, Z = 4, μ(CuKα) = 0.687 mm−1, 6450 reflections measured, 3642 independent reflections (R int = 0.0501). The final R 1 values were 0.0653 (I > 2σ(I)). The final wR(F 2) values were 0.1771 (I > 2σ(I)). The final R 1 values were 0.0661 (all data). The final wR(F 2) values were 0.1777 (all data). The goodness of fit on F 2 was 1.167. Flack parameter = 0.0 (4). The Hooft parameter is 0.03 (17) for 1371 Bijvoet pairs. Crystallographic data for the structure of 1 have been deposited in the Cambridge Crystallographic Data Centre (deposition number CCDC 1528224). Copies of the data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk.
Schisanpropinin (2): yellowish solid, \(\left[ \alpha \right]_{\text{D}}^{20}\) −31.2 (c 0.11, MeOH), UV (MeOH) λ max (log ε) 286 (4.06), 205 (4.96) nm, IR (KBr) v max 3424, 2928, 2856, 1607, 1518, 1463, 1431, 1383, 1277, 1160, 1071, 1031, 785 cm−1. ESIMS m/z 389 [M − H]−, HRESIMS m/z 389.1237 [M − H]− (calcd for C20H21O8 389.1242). 1H NMR (C5D5N, 600 MHz) and 13C NMR (C5D5N, 150 MHz), see Table 1.
References
Y.W. Law, Flora Repubulicae Popularis Sinicae, vol. 30 (Science Press, Beijing, 1996), pp. 231–273
W.L. Xiao, R.T. Li, S.X. Huang, J.X. Pu, H.D. Sun, Nat. Prod. Rep. 25, 871–891 (2008)
R.T. Li, Q.S. Zhao, S.H. Li, Q.B. Han, H.D. Sun, Y. Lu, L.L. Zhang, Q.T. Zheng, Org. Lett. 5, 1023–1026 (2003)
Y.M. Shi, W.L. Xiao, J.X. Pu, H.D. Sun, Nat. Prod. Rep. 32, 367–410 (2015)
C. Lei, S.X. Huang, J.J. Chen, L.B. Yang, W.L. Xiao, Y. Chang, Y. Lu, H. Huang, J.X. Pu, H.D. Sun, J. Nat. Prod. 71, 1228–1232 (2008)
C. Lei, J.X. Pu, S.X. Huang, J.J. Chen, J.P. Liu, L.B. Yang, Y.B. Ma, W.L. Xiao, X.N. Li, H.D. Sun, Tetrahedron 65, 164–170 (2009)
C. Lei, S.X. Huang, J.X. Pu, J.P. Liu, G.Y. Yang, L.B. Yang, Y. Lu, J.J. Chen, Y. Lu, W.L. Xiao, H.D. Sun, Planta Med. 74, 292–295 (2008)
C. Lei, S.X. Huang, J.J. Chen, J.X. Pu, L.B. Yang, Y. Zhao, J.P. Liu, X.M. Gao, W.L. Xiao, H.D. Sun, Chem. Pharm. Bull. 55, 1281–1283 (2007)
M. Liu, J. Wan, Y.Q. Luo, X.N. Li, X. Du, J.X. Pu, H.D. Sun, Nat. Prod. Commun. 11, 925–929 (2016)
M.W. Lodewyk, C. Soldi, P.B. Jones, M.M. Olmstead, J. Rita, J.T. Shaw, D.J. Tantillo, J. Am. Chem. Soc. 134, 18550–18553 (2012)
Z.X. Hu, Y.M. Shi, W.G. Wang, J.W. Tang, M. Zhou, X. Du, Y.H. Zhang, J.X. Pu, H.D. Sun, Org. Lett. 18, 2284–2287 (2016)
A. Chandra, L.N. Misra, R.S. Thakur, Phytochemistry 26, 3077–3078 (1987)
R.M. Coates, J.F. Denissen, J.A. Juvik, B.A. Babka, J. Org. Chem. 53, 2186–2192 (1988)
H.D. Flack, Acta Crystallogr. A 39, 876–881 (1983)
R.W.W. Hooft, L.H. Straver, A.L. Spek, J. Appl. Crystallogr. 41, 96–103 (2008)
K.S. Kulkarni, S.K. Paknikar, A.S. Vaidya, G.R. Kelkar, R.B. Bates, S.C. Bhattacharyya, Tetrahedron Lett. 8, 505–511 (1963)
N.B. Perry, E.J. Burgess, L.M. Foster, P.J. Gerard, Tetrahedron Lett. 44, 1651–1653 (2003)
N.B. Perry, E.J. Burgess, L.M. Foster, M. Toyota, Y. Asakawa, J. Nat. Prod. 71, 258–261 (2008)
M. Massias, S. Rebuffat, L. Molho, A. Chiaroni, C. Riche, B. Bodo, J. Am. Chem. Soc. 112, 8112–8115 (1990)
Y.S. Che, J.B. Gloer, B. Koster, D. Malloch, J. Nat. Prod. 65, 916–919 (2002)
Z. Guo, F.X. Ren, Y.S. Che, G. Liu, L. Liu, Molecules 20, 14611–14620 (2015)
H. Oh, J.B. Gloer, C.A. Shearer, J. Nat. Prod. 62, 497–501 (1999)
F.A. Macias, R.M. Varela, A.M. Simonet, H.G. Cutler, S.J. Cutler, R.A. Hill, Tetrahedron Lett. 44, 941–943 (2003)
L.H. Zhang, B.M. Feng, G. Chen, S.G. Li, Y. Sun, H.H. Wu, J. Bai, H.M. Hua, H.F. Wang, Y.H. Pei, RSC Adv. 6, 42361–42366 (2016)
Y.M. Ying, C.A. Fang, F.Q. Yao, Y. Yu, Y. Shen, Z.N. Hou, Z. Wang, W. Zhang, W.G. Shan, Z.J. Zhan, Chem. Biodivers. (2017). doi:10.1002/cbdv.201600184
Y. Wang, D.H. Li, Z.L. Li, Y.J. Sun, H.M. Hua, T. Liu, J. Bai, Molecules 21, 31–36 (2016)
J.X. Pu, X.M. Gao, C. Lei, W.L. Xiao, R.R. Wang, L.B. Yang, Y. Zhao, L.M. Li, S.X. Huang, Y.T. Zheng, H.D. Sun, Chem. Pharm. Bull. 56, 1143–1146 (2008)
A. Monks, D. Scudiero, P. Skehan, R. Shoemaker, K. Paull, D. Vistica, C. Hose, J. Langley, P. Cronise, A. Vaigro-Wolff, M. Gray-Goodrich, H. Campbell, J. Mayo, M. Boyd, J. Natl. Cancer 83, 757–766 (1991)
M. Zhou, K. Zhou, X.M. Gao, Z.Y. Jiang, J.J. Lv, Z.H. Liu, G.Y. Yang, M.M. Miao, C.T. Che, Q.F. Hu, Org. Lett. 17, 2638–2641 (2015)
M. Zhou, M.M. Miao, G. Du, X.N. Li, S.Z. Shang, W. Zhao, Z.H. Liu, G.Y. Yang, C.T. Che, Q.F. Hu, X.M. Gao, Org. Lett. 16, 5016–5019 (2014)
L.J. Reed, H. Muench, Am. J. Hyg. 27, 493–497 (1938)
Acknowledgements
This project was supported financially by the National Natural Science Foundation of China (81373290 and 21322204).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, M., Hu, ZX., Luo, YQ. et al. Two New Compounds from Schisandra propinqua var. propinqua . Nat. Prod. Bioprospect. 7, 257–262 (2017). https://doi.org/10.1007/s13659-017-0129-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13659-017-0129-7