Abstract
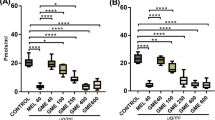
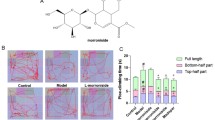
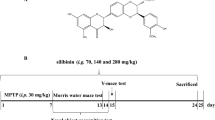
Parkinson’s disease (PD) is a progressive degenerative disorder of the central nervous system (CNS) that leads to impairment of motor skills and speech. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) causes damage to the dopaminergic (DA) neurons, and 1-4-Methyl-4-phenylpyridinium (MPP+) causes cell death in differentiated PC12 cells that is similar to the degeneration that occurs in PD. Moreover, MPTP treatment increases the activity of the microglia cells that produced reactive oxygen species (ROS). We recently reported that Ligustri Fructus (LF), a widely used traditional herbal medicine, increases cell viability in a yeast model of PD. In the present study, we examined the inhibitory effect of LF (0.01, 5, 10 ug) on the neurotoxicity of MPTP in mice and on the MPP + -induced cell death in differentiated PC12 cells. In vivo experiment, MPTP injection revealed a significant loss of DA neurons in the substantia nigra, while LF (100, 200 mg/ kg) treatment dramatically reversed DA neuron loss in immunohistochemistry assay for tyrosine hydroxylase (TH). Furthermore, LF attenuated the MPP + -induced cell death, decreased the generation of ROS, and activated glutathione peroxidase in PC12 cells. These results suggest that LF may be beneficial for the treatment of neurodegenerative diseases such as PD.




Similar content being viewed by others
References
Bao X, Wang Z, Fang J, Li X (2002) Structural features of an immunostimulating and antioxidant acidic polysaccharide from the seeds of Cuscuta chinensis. Planta Med 68:237–243. doi:10.1055/s-2002-23133
Beal MF (2002) Oxidatively modified proteins in aging and disease. Free Radic Biol Med 32:797–803
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69. doi:10.1038/nrn2038
Chiueh CC, Krishna G, Tulsi P, Obata T, Lang K, Huang SJ, Murphy DL (1992) Intracranial microdialysis of salicylic acid to detect hydroxyl radical generation through dopamine autooxidation in the caudate nucleus: effects of MPP+. Free Radic Biol Med 13:581–583
Choi DK et al (2005) Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson’s disease in mice. J Neurosci 25:6594–6600. doi:10.1523/JNEUROSCI.0970-05.2005
Chung YC et al (2011) Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology 60:963–974. doi:10.1016/j.neuropharm.2011.01.043S0028-3908(11)00054-2
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Dexter DT, Jenner P (2013) Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 62:132–144. doi:10.1016/j.freeradbiomed.2013.01.018
Fahn S, Cohen G (1992) The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol 32:804–812. doi:10.1002/ana.410320616
Feng LR, Maguire-Zeiss KA (2010) Gene therapy in Parkinson’s disease: rationale and current status. CNS Drugs 24:177–192. doi:10.2165/11533740-000000000-000001
Gao HM, Liu B, Zhang W, Hong JS (2003) Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J 17:1954–1956. doi:10.1096/fj.03-0109
Gao L, Li C, Wang Z et al. (2014) Ligustri Lucidi Fructus as a traditional Chinese medicine: a review of its phytochemistry and pharmacology. Nat Prod Res:1-18 doi: 10.1080/14786419.2014.954114
Haleagrahara N, Ponnusamy K (2010) Neuroprotective effect of Centella asiatica extract (CAE) on experimentally induced parkinsonism in aged Sprague-Dawley rats. J Toxicol Sci 35:41–47
Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP (2003) The role of glial reaction and inflammation in Parkinson’s disease. Ann N Y Acad Sci 991:214–228
Kadota T et al (1996) Expression of dopamine transporter at the tips of growing neurites of PC12 cells. J Histochem Cytochem 44:989–996
Koutsilieri E, Scheller C, Tribl F, Riederer P (2002) Degeneration of neuronal cells due to oxidative stress--microglial contribution Parkinsonism. Relat Disord 8:401–406
Lang AE, Lozano AM (1998) Parkinson’s disease. first of two parts. N Engl J Med 339:1044–1053. doi:10.1056/NEJM199810083391506
Langston JW, Irwin I, Langston EB, Forno LS (1984) 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neurosci Lett 48:87–92
Li X, Li Y, Chen J, Sun J, Sun X, Kang X (2010) Tetrahydroxystilbene glucoside attenuates MPP + -induced apoptosis in PC12 cells by inhibiting ROS generation and modulating JNK activation. Neurosci Lett 483:1–5. doi:10.1016/j.neulet.2010.07.027S0304-3940(10)00928-6
Liberatore GT et al (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5:1403–1409. doi:10.1038/70978
Liu JH, Ho SC, Lai TH, Liu TH, Chi PY, Wu RY (2003) Protective effects of Chinese herbs on D-galactose-induced oxidative damage. Methods Find Exp Clin Pharmacol 25:447–452
McGeer PL, McGeer EG (2008) Glial reactions in Parkinson’s disease. Mov Disord 23:474–483. doi:10.1002/mds.21751
Miller RL, James-Kracke M, Sun GY, Sun AY (2009) Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res 34:55–65. doi:10.1007/s11064-008-9656-2
Ogawa N (1997) Dopamine neurotransmission and treatments for Parkinson’s disease in the molecular biology era. Eur Neurol 38(Suppl 1):2–5
Pereira CF, Oliveira CR (2000) Oxidative glutamate toxicity involves mitochondrial dysfunction and perturbation of intracellular Ca2+ homeostasis. Neurosci Res 37:227–236
Qin DN, She BR, She YC, Wang JH (2000) Effects of flavonoids from Semen Cuscutae on the reproductive system in male rats. Asian J Androl 2:99–102
Riederer P, Wuketich S (1976) Time course of nigrostriatal degeneration in parkinson’s disease. a detailed study of influential factors in human brain amine analysis. J Neural Transm 38:277–301
Santos CM (2012) New agents promote neuroprotection in Parkinson’s disease models. CNS Neurol Disord Drug Targets 11:410–418
Sung SH, Kim ES, Lee KY, Lee MK, Kim YC (2006) A new neuroprotective compound of Ligustrum japonicum leaves. Planta Med 72:62–64. doi:10.1055/s-2005-873140
Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, Banerjee R (2006) A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem 281:35785–35793. doi:10.1074/jbc.M602799200
Wang SS, Chen JH, Liu XJ (1994) Preliminary study on pharmacologic action of Ligustrum japonicum. Zhongguo Zhong Xi Yi Jie He Za Zhi 14:670–672
Wang Z, Fang JN, Ge DL, Li XY (2000) Chemical characterization and immunological activities of an acidic polysaccharide isolated from the seeds of Cuscuta chinensis. Lam Acta Pharmacol Sin 21:1136–1140
Wang XM, Fu H, Liu GX (2001) Effect of wuzi yanzong pill and its disassembled prescription on mitochondrial DNA deletion, respiratory chain complexes and ATP synthesis in aged rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 21:437–440
Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S (2003) NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci U S A 100:6145–6150. doi:10.1073/pnas.0937239100
Yang HM, Shin HK, Kang YH, Kim JK (2009) Cuscuta chinensis extract promotes osteoblast differentiation and mineralization in human osteoblast-like MG-63 cells. J Med Food 12:85–92. doi:10.1089/jmf.2007.0665
Zeevalk GD, Bernard LP, Albers DS, Mirochnitchenko O, Nicklas WJ, Sonsalla PK (1997) Energy stress-induced dopamine loss in glutathione peroxidase-overexpressing transgenic mice and in glutathione-depleted mesencephalic cultures. J Neurochem 68:426–429
Zhang ZT, Cao XB, Xiong N, Wang HC, Huang JS, Sun SG, Wang T (2010) Morin exerts neuroprotective actions in Parkinson disease models in vitro and in vivo. Acta Pharmacol Sin 31:900–906. doi:10.1038/aps.2010.77
Zhang JF et al (2011) Aqueous extracts of Fructus Ligustri Lucidi enhance the sensitivity of human colorectal carcinoma DLD-1 cells to doxorubicin-induced apoptosis via Tbx3 suppression. Integr Cancer Ther 10:85–91. doi:10.1177/1534735410373921
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Education, Science, and Technology, MEST) (no. 2007-0054931).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statements
The study was approved by the University of Kyung Hee Animal Care and Use Committee (KHUASP(SE)-11-010).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Ye, M., Kim, M. & Bae, H. Neuro-protective effects of Ligustri Fructus by suppression of oxidative stress in mouse model of Parkinson’s disease. Orient Pharm Exp Med 16, 123–129 (2016). https://doi.org/10.1007/s13596-016-0223-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-016-0223-0