Abstract
Key message
Abies fargesii var. faxoniana (Rehder et E.H. Wilson) Tang S Liu seedlings at high elevations compensate for the low efficiency of their water conducting system and high phloem hydraulic resistance by the enhancement in xylem:leaf area, phloem:leaf area, and phloem:xylem area.
Context
Maintenance of xylem and phloem transport is particularly important for the survival and growth of trees at the treeline. How plants modify the allocation to leaf, xylem, and phloem structures to adapt to the treeline environment is an important issue.
Aims
The purpose of this study was to estimate how xylem and phloem anatomy and volume as well as leaf functional traits of A. fargesii seedlings vary with elevation.
Methods
We examined elevation-related differences in a variety of phloem and xylem functional areas and hydraulic conduit diameters of A. fargesii seedlings growing at elevations between 2600 and 3200 m in the subalpine conifer forest of southwest China.
Results
Xylem area, last xylem ring area, and leaf:sapwood area significantly decreased, while xylem:leaf area, phloem:leaf area, and non-collapsed phloem:xylem area significantly increased with elevation. Principal components analysis showed that xylem area, non-collapsed phloem area, and xylem:phloem area were positively correlated with growth rates.
Conclusion
Our results showed that A. fargesii tree seedlings at the treeline tend to facilitate growth and maintain functional water and sugar balance between stem and leaves by the enhancement in xylem:leaf area, phloem:leaf area, and phloem:xylem area, but not through differences in vessel lumen diameter.
Similar content being viewed by others
1 Introduction
Treelines are dynamic and have shifted in the past, and the ecological processes and climatic determinants of the treeline are not well understood. At the moment, two main hypotheses attempt to explain treeline location on a global scale. The “source limitation” hypothesis assumes that low temperatures and short growing seasons limit the rates of CO2 acquisition (photosynthesis) or the rates of nutrient mineralization and uptake (McNown and Sullivan 2013). As an alternative, Körner (1998) proposed the “growth limitation” hypothesis, which states that low temperatures limit the rates of cell division and tissue formation from meristems. To date, many studies have found support for the growth limitation hypothesis in various treelines (Wiley and Helliker 2012; Dawes et al. 2015), while others favor the source limitation idea (e.g., Susiluoto et al. 2010). The growth limitation assumes implicitly that plant anatomical structures are crucial for tree performance in mountain regions (Rossi et al. 2008). However, the existing theories on treeline formation still lack evidence from the xylem and phloem anatomy of trees at the treeline (Losso et al. 2018; Mayr et al. 2019).
Sources and sinks are linked to each other through the coupled xylem and phloem transport systems (Steppe et al. 2015; Růžička et al. 2015; Savage et al. 2016; Sevanto et al. 2018). Xylem is responsible for water and solute transport from the roots to the leaves. Water transport in the xylem is a passive process, driven by the water potential difference between roots and leaves. In contrast, phloem transport, responsible for sugar transport from the leaves to the roots, is based on active loading of the phloem driven by osmotic pressure as proposed by Münch pressure flow hypothesis (Münch 1930). At high elevations, low temperatures delay the xylem transport due to the reduced leaf-to-soil water potential difference. Low temperatures also limit phloem transport either due to increased viscosity of the sugary solution or decreased hydrostatic pressure gradient along the phloem (Hölttä et al. 2006). Adaptations to low temperature may lead to changes in xylem and phloem structures. Narrower xylem conduits and lower leaf to sapwood area ratio have been reported in trees at high compared to low elevations (Mayr et al. 2007; Creese et al. 2011). Xylem:leaf area and phloem:leaf area are a proxy for the balance of water supply versus water loss, and the balance between sugar transport versus sugar production (Zhang et al. 2016, 2020). In this context, we expect that plants at high elevations produce more xylem area and phloem area for a given leaf area, in an attempt to maintain functional water and sugar balance between stem and leaves. This hypothesis still needs to be tested.
We here present a conceptual model (Fig. 1) that claims that trees at the treeline might express adaptive traits in order to maintain efficient water and sugar transport under stressful environmental conditions. Trees are generally exposed to water stress at the timberline, possibly induced by lower soil temperatures at higher altitudes—which in turn may block root water uptake (Mayr et al. 2019). Moreover, a combination of water stress and high vapor pressure deficit (VPD) induced by strong winds enhances evaporative demands on trees at treeline, which is expected to further increase potential transpiration rates, resulting in negative leaf water potential at high elevations. Because transpiration from the leaves creates a water potential gradient that drives xylem water transport, net translocation of sugars in the phloem must work against this overall gradient created by transpiration (Hölttä et al. 2006). As described earlier, phloem transport capacity depends on xylem water potential. The higher the xylem tension (more negative the water potential), the more difficult sugar transport is (Savage et al. 2016). Moreover, the viscosity of phloem sap is likely to increase at low temperate (Hölttä et al. 2009). Consequently, phloem transport capacity at high elevation can be limited either by increasing sap viscosity or a decrease in turgor pressure during high xylem water tension (Cernusak et al. 2003; Hölttä et al. 2009). In this context, plants could compensate for the low leaf water potentials (minimize xylem tensions) through phenotypic adjustments such as increases in water use efficiency, decreases in foliage to sapwood area, and differences in xylem structural traits (Fig.1). Similarly, acclimation of hydraulic conductivity and plant water relations to high elevations, have been described previously (Wan et al. 2001; Li et al. 2004; Poorter et al. 2010; Pritzkow et al. 2014). We predict that, close to the treeline, trees also adjust their phloem traits to increase the capacity of the phloem to transport photosynthates to the sinks (Fig. 1). These acclimations would be the increases in phloem tissue or the high allocation of phloem per leaf area to offset increases in phloem sap viscosity. So far, this reasoning still lacks a direct test.
Conceptual model of phloem and xylem transport and the traits that affect the transport. Blue arrows show negative effects, and red arrows show positive effects on transport. Reductions in xylem tensions, as caused by most adaptations to soil drying by freezing, facilitate the phloem transport because phloem transport occurs against the hydrostatic pressure gradient, while xylem transport is with the hydrostatic gradient. Trees at the treeline require large functional xylem and phloem areas to compensate for the low water transport ability of xylem and high phloem sap viscosity at the treeline
Abies fargesii var. faxoniana (Rehder & E. H. Wilson) Tang S. Liu, an evergreen alpine and subalpine endemic conifer species, is widely distributed in the Sichuan province, Southwest China. It plays a very important role in preventing soil erosion and in retaining ecological stability (Wang et al. Wang 2004). In this study, we analyzed leaf:sapwood area, specific leaf area, xylem and phloem anatomy, and growth of A. fargesii seedlings growing along an elevational transect of 600 m. We focused on seedlings because they have a smaller and shallower root system than mature trees and are therefore more susceptible to soil drying by freezing than conspecific adult trees. Our aims were to estimate how xylem and phloem anatomy, leaf functional traits of A. fargesii tree seedlings vary with elevations. We hypothesized that seedlings at the treeline have larger xylem and phloem area fractions to compensate for the low conductivity because of the low water transport ability of the xylem, high phloem sap viscosity and associated high resistance in cold soils.
2 Materials and methods
2.1 Study area and sampling
The study area is located in the Wanglang Nature Reserve (32°49′ – 33°02’N, 103°55′ – 104°10′E, 2300–4980 m above sea level), Southwest China. The treeline in the area is approximately 3600 m. This region has wet, cool summers and dry, cold winters. We selected trees at three elevations along a 600-m elevation transect, beginning at 2600 m and extending up to 3200 m (Table 1). The selected transect was southeast exposed. Our study plots at 2600 m were situated on a slope of 15°, but at 2900 m and 3200 m the slope was 25°. Weather data at the three elevation areas were collected from nearby meteorological stations belonging to the Wanglang Nature Reserve Agency. From 2600 m to 3200 m, the mean annual air temperature drops from 4.4 to 1.5 °C, and the mean annual precipitation ranges from 700 to 900 mm. In winter, the depth of the frozen soil is over 40 cm. Soils are mountain dark brown coniferous forest soils. The dominant canopy trees for the three altitude plots are A. fargesii. The stand densities were 578, 602, and 403 trees ha−1 at 2600 m, 2900 m, and 3200 m altitude, respectively. Stands at 2600 m have an average height of 17.0 m and an average DBH of 40.0 cm, stands at 2900 m have an average height of 12.0 m and an average DBH of 27.0 cm, and stands at 3200 m have an average height of 5.0 m and an average DBH of 6.5 cm. The duration of the growing season lasted from mid-May to the end of August, with the length of the growing season 109 days in 2015. Samples were collected randomly from ten distinct sites at each elevation, which were considered replications. Individuals of similar age (5–7 years) were selected (10 individuals at each elevation, a total of 30 individuals). All samples of branches and needles were collected from tree seedlings in stands within a very limited geographic range, and only from sun-exposed tops of tree seedlings to standardize for the effects of inclination, irradiance, soil characteristics, and microclimate. A pyranometer in the 300–1150 nm range (SK01-D, Australia) with an accuracy of ±3% was used to measure the incoming total solar radiation. During the growing season (from June to September), the mean daily solar radiation of forest understory was 65.74 ± 45.06 MJ m−2 d−1 at the elevation of 2600 m, 60.37 ± 45.71 MJ m−2 d−1 at the elevation of 2900 m, and 67.23 ± 39.96 MJ m−2 d−1 at the elevation of 3200 m, respectively.
We sampled needles and branches at the end of the growing season in order to ensure that the formation of xylem and phloem had ceased. Anatomical analyses were performed on randomly selected samples (10 branches per elevation) collected from trees of similar height at three different elevations (2600, 2900, and 3200 m) in September 2015. The accuracy of seedlings’ age was assessed from node counts in the field following a standardized protocol (Hankin et al. 2018). Annual nodes were identifiable by bud scars. The ages of leaves and branches were determined by using marks from over-wintering buds on the stem, stepwise changes in needle anatomy, and by counting back the internodes from the tip of the stems.
2.2 Seedling growth and sapwood area measurements
Ten individuals aged between 5 and 7 years from each altitude were selected for seedling growth measurements. The height and root collar diameter of the same sample seedlings were measured with a ruler and a digital vernier caliper, respectively. Total height was measured from the base to the terminal shoot apex to the nearest 1 mm. The basal diameter was measured with a caliper to the nearest 0.1 mm. Height increments were determined by measuring the distance from the apex to the most recent resting bud scar, and growth rates are expressed based on height increments. For each branch of each seedling, all leaves were collected to determine the total leaf area, leaf mass, and specific leaf area (SLA, the leaf area:leaf mass). The seedling was harvested. Leaf area (cm2) was measured on 100 needles per tree and 10 tree seedlings per altitude on images made using a flatbed scanner. After all leaves were scanned for leaf area measurements, they were oven-dried at 70 °C for 48 h in order to determine the specific leaf area. Total leaf area per branch segment was calculated by dividing dry weight through specific leaf area values. The cross-sectional area of the sapwood was measured with digital calipers and was assumed to be the total cross-section of the main stem after the bark was removed. The leaf:sapwood area was calculated by dividing the total leaf area by the corresponding sapwood area.
2.3 Phloem and xylem anatomical measurements
For the measurements of xylem and phloem tissue areas and cellular dimensions, and for the further calculations on xylem:leaf area and phloem:leaf area, we cut a branch section of 10 cm length at a fixed 5-cm distance from the apex of each tree seedling. At each elevation, 10 branches from 10 tree seedlings were collected for anatomical analyses. To preserve the structures of phloem and xylem for detailed microscopy analysis, the branch samples were preserved in a 50% solution of ethanol–water directly after sampling in the field. In the laboratory, the samples (5-cm distance from the apex) were then cut into smaller specimens, dehydrated, and embedded into plastic resin blocks. Blocks were further cut into thin sections, and directed for light microscopy analysis according to the procedures described below.
The ethanol preserved branch samples were cut into smaller specimens (8 × 3 × 3 mm, radial × tangential × longitudinal), including either xylem with cambial zone, or cambial zone with non-collapsed phloem, collapsed phloem, and outer bark. These overlapping specimens covered one representative radius of each branch sample that was selected based on visual observation, avoiding compression wood, opposite wood, or other damages or abnormalities. The specimens were then dehydrated in a graded series of ethanol and ethanol–resin mixture (LR White Resin, medium grade, London Resin Co., Ltd., London, UK), and then embedded into resin blocks and transverse cross-sections (ca. 5–8 μm thick) were cut with a rotary microtome (Leica RM2265, Leica Microsystems, Wetzlar, Germany) using glass knives. The sections were stained with an aqueous solution of toluidine blue (AppliChem GmbH, Darmstadt, Germany), air dried, and permanently mounted on objective slides using Ultrakitt M540 mountant (TAAB, Reading, UK).
Images of the cross-sections of the branch specimens were taken using a digital camera (MicroPublisher 3.3 RTV, QImaging, Surrey, Canada) attached to a light microscope (Olympus BX60, Olympus, Tokyo, Japan) at a 40X magnification (resolution 0.085 μm pixel−1). One image was used or several overlapping images were joined to cover the whole area of the tissues of interest. These images were analyzed with IMAGE-PRO PLUS v.7.0. (Media Cybernetics Inc., Bethesda, MD, USA).
From the images, the total widths of xylem and bark, the latest xylem annual ring, non-collapsed phloem, and collapsed phloem were measured along with three radial locations in each cross-section by using analysis software (IMAGE-PRO PLUS v. 7.0., Media Cybernetics Inc., Bethesda, MD, USA). Tissues were identified on the basis of their morphology and color (see Jyske and Hölttä 2015), i.e., the non-collapsed phloem was identified as a narrow strip outside the cambium consisting of un-collapsed, conducting (i.e., functional) sieve cells. Sieve cells were slightly larger in size (both lumen and cell wall thickness in radial direction) as compared to the cambial cells. Collapsed phloem was identified based on the changed (collapsed) morphology of sieve cells after they had lost the turgor and functionality in phloem transport. The corresponding tissue areas of the xylem and phloem were further calculated by using geometry and assuming circular branch cross-sections.
For the measurements of xylem and phloem cellular dimensions, the areas of the last-formed xylem ring and the non-collapsed phloem were selected by a cropping tool (Paint, Microsoft Windows, version 6.1). The whole tissue area including all the cells within the tissue type in each cross-section was selected for the cropped area, avoiding any fractured cells and areas. The cropped image areas were then analyzed for the average size of xylem and phloem cells (i.e., cell lumen diameter, cell wall thickness) by using an automated image analysis method (see Antikainen 2017 for a detailed description of the analysis method) using the Matlab Image Processing Toolbox.
2.4 Analyses of leaf water potential, carbon isotope composition, tissue carbohydrates, and foliar nitrogen
The measurements of leaf water potential, foliar carbon isotope discrimination (δ13C) and N concentration, and non-structural carbohydrate (NSC) were sampled at the same time of the day (10:00–11:00) to avoid diurnal variation. All of the dried leaf samples were ground into fine powder through a mesh (pore diameter ca. 275 μm) and then used to analyze the needles N content, foliar signature of δ13C, the concentrations of NSC in leaves and branches (current- and previous-year separately), and roots. The midday leaf water potential was determined using a WP4 Dewpoint Potentiometer (Decagon Devices, Inc., Pullman, WA, USA). The concentrations of N in leaves were determined by the semi-micro Kjeldahl method (Mitchell 1998). The abundance of stable carbon isotopes in the leaf samples was determined following Hubick et al. (1986). Oven-dried samples were finely ground, and the relative abundance of 13C and 12C was determined with an isotope ratio mass spectrometer (Finnegan MAT Delta-E). δ13C was expressed relative to the PeeDee Belemnite standard (Craig 1957). The overall precision of the values was better than 0.1 ‰, as determined by repetitive samples. Soluble sugar and starch concentrations were extracted from c. 20 mg of fine powder in 5 ml of 80% aqueous ethanol (v:v). The mixture was boiled in a water bath at 80 °C for 30 min, and then centrifuged at 5000 g for 10 min. The supernatant was used for the determination of total soluble sugar content with a spectrophotometer at 490 nm (model UV-PC01; Shimadzu Corp., Kyoto, Japan). Starch in the residue was released by boiling the residue from the ethanol extraction in 3% HCl (w/w, 0.08 N) for 3 h. Starch concentration was calculated as the sugar concentration multiplied by the conversion factor of 0.9 (Osaki et al. 1991). Glucose was used as a standard. NSC concentration was defined as the sum of the starch and the soluble sugars for each sample in this study.
2.5 Statistical analysis
Analyses of covariance (ANCOVA) were performed with the software package SPSS 20.0 (SPSS Inc., Chicago, IL) to test the fixed effects of elevation using the general linear model with growth rate as a covariate. All traits were natural-log-transformed to meet the assumptions of ANCOVA. The altitude effect persisted after accounting for differences in growth rates, demonstrating that the statistical effects of the elevational trend in leaf morphological traits, anatomical traits, xylem and phloem traits, and functional ratio traits observed in our study are independent of growth rates. When elevation has a significant effect on a variable, means were compared for significant differences by Tukey’s multiple-comparison method. Generalized linear regression analysis was calculated to provide supplementary information on the relationship between functional areas of xylem and phloem. A one-way ANCOVA was used to detect differences among elevations in the relationships between different variables. Principal-component analysis (PCA) was done to evaluate how growth rate and wood anatomy are associated with each other and to characterize the variability among wood anatomy, functional ratio traits, and plant growth among the three elevation sites. The software package CANOCO 5.0 for Windows was used for the PCA analyses. The growth data were log-transformed to have a standard deviation of one and mean zero. The significance of the ordination analysis was tested by using Monte Carlo permutation tests (499 unrestricted permutations, P < 0.05) (Ter Braak and Smilauer 2002).
3 Results
3.1 Leaf water potential, plant growth, functional ratio, and leaf traits
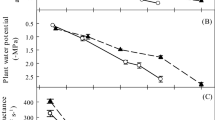
Midday leaf water potential was significantly lower at higher altitudes (P < 0.05, Fig. 2a). Leaf δ13C (water use efficiency) was significantly different among altitudes, with the greatest and lowest values at 3200 m and 2900 m, respectively (Fig. 2b). Tree heights ranged from 44 to 59 cm, and the main stem diameter varied from 11 to 13 mm (Fig. 2c, d). The main stem diameter, height, xylem:leaf area, phloem:leaf area, and specific leaf area displayed large variations. The main stem diameter was similar at 2600 m and 2900 m, but was significantly higher at 3200 m (P < 0.05, Fig. 2c). Xylem:leaf area and phloem: leaf area significantly increased (P < 0.05, Fig. 2g, i), while height, leaf:sapwood area, and specific leaf area significantly decreased with increasing altitude (P < 0.05, Fig. 2d–f). The leaf N contents did not vary with altitude (P > 0.05, Fig. 2h).
Leaf water potential(a), leaf isotope discrimination (δ13C)(b), stem diameter (c), height (d), leaf:sapwood area (e), specific leaf area (f), phloem:leaf area (g), leaf N contents (h), and xylem:leaf area (i) of A. fargesii seedlings at 2600 m, 2900 m, and 3200 m altitudes (n = 10 branches per elevational site). Boxplots show the median (horizontal bar), interquartile range (box), range (lines), and outliers (points). Mean values are indicated by small square. Different letters indicate significant differences (P < 0.05) among elevations according to Tukey’s multiple-comparisons method
3.2 Phloem and xylem anatomical characteristics
The xylem conduit diameter was similar at 2600 m and 2900 m, but was significantly lower at 3200 m (Fig. 3a). Xylem area and last xylem ring area decreased while non-collapsed phloem: xylem area and non-collapsed phloem: last xylem ring area increased significantly with increasing elevation (P < 0.05, Fig. 3b–f). The non-collapsed phloem area at 2900 m was higher (P < 0.05) than at 3200 m (Fig. 3c). In contrast, phloem and xylem cell size and cell wall thickness did not change significantly with elevation (P > 0.05, Fig. 4a, b).
Xylem conduit diameter (a), xylem area (b), non-collapsed phloem area (c), last xylem ring area (d), non-collapsed phloem: xylem area (e), and non-collapsed phloem:last xylem ring area (f) of A. fargesii seedlings at 2600 m, 2900 m and 3200 m altitudes (n = 10 branches per elevational site). Boxplots show the median (horizontal bar), interquartile range (box), range (lines), and outliers (points). Mean values are indicated by small square. Different letters indicate significant differences (P < 0.05) among elevations according to Tukey’s multiple-comparisons method
Cell wall thickness (a) and cell lumen diameter (b) of the xylem conduits in A. fargesii seedlings at 2600 m, 2900 m, and 3200 m altitudes (n = 10 branches per elevational site). Boxplots show the median (horizontal bar), interquartile range (box), range (lines), and outliers (points). Mean values are indicated by small square. P values and significance levels (ANOVA): Pa, altitude effect
3.3 Correlations between functional areas of xylem and phloem
Log–log bivariate regression analysis showed that non-collapsed phloem area scaled with the xylem area in each elevation tested (R2 = 0.66 for 2600 m, R2 = 0.38 for 2900, R2 = 0.71 for 3200 m, Fig. 5a). We also found significant positive correlations between non-collapsed phloem area and last xylem ring area (R2 = 0.43 for 2600 m, R2 = 0.48 for 2900, R2 = 0.90 for 3200 m, Fig. 5b). When the log non-collapsed phloem area was plotted against the log xylem area and the last xylem ring area, there was no significant difference in the regression line among elevations (Fig. 5a, b, ANOVA, P > 0.05).
3.4 PCA analysis
PCA revealed that the first two components accounted for 86.2% of the total variation among the 12 variables (Fig. 6). The first axis (Ax1) explained 67.6% of the variance, the second axis (Ax2) explains 18.6% of the variation. The first axis was mainly correlated with traits related to xylem area, last xylem ring area, and xylem:phlom area. The second axis was mainly associated with traits related to growth rate, δ13C, and non-collapsed phloem area. The growth rate was positively correlated with non-collapsed phloem area (r = 0.674; P < 0.01), xylem area (r = 0.640; P < 0.01), and xylem:phloem area (r = 0.547; P < 0.01) (Fig. 6).
Principal component analysis on tree seedling growth rate and leaf traits, anatomical traits, xylem and phloem traits, and functional ratio traits of A. fargesii at 2600 m, 2900 m, and 3200 m altitudes. Ψ, leaf water potential; δ13C, leaf isotope discrimination; Pcld, Phloem cell lumen diameter; Xcld, xylem cell lumen diameter; Xa, xylem area fraction; Lxa, last xylem ring area fraction; Npa, non-collapsed phloem area fraction; PL, phloem: leaf area; XL, xylem:leaf area; LS, leaf:sapwood;XP, xylem:phloem area; GR, growth rate
3.5 Nonstructural carbohydrate (NSC)
All tissues had higher concentrations of starch than of low-molecular-weight sugars. Concentrations of soluble sugars, fructose, sucrose, total soluble sugar, and NSC in needles, branches, and roots did not decrease with increasing altitude (Fig.7). There were no significant trends in sugar, fructose, sucrose, and starch concentrations in needle, branch, and root tissues with increasing altitude (P > 0.05).
Concentration of starch (a), sucrose (b), fructose(c), total soluble sugars (d) and NSC (the sum of free sugars and starch) (e) in leaves and branches (previous year, white; current year, gray) and roots of A. fargesii seedlings at 2600 m, 2900 m, and 3200 m altitudes. Values are mean ± SE for 10 trees seedlings. P values and significance levels (ANOVA): Pa, altitude effect
4 Discussion
In accordance with our hypothesis, A. fargesii seedlings at high elevations had a lower SLA and higher xylem area per unit leaf area than did trees at lower elevations. This confirms that trees compensate for their less efficient water-conducting system by producing more xylem for a given leaf area. Furthermore, in the present study, the xylem area decreased significantly while the non-collapsed phloem:xylem area and the phloem:leaf area increased significantly with increasing altitudes. This supports our hypotheses that increasing limitations of tree growth are due to limitations of phloem transport in high altitudes, and trees at high altitudes exhibit structural adaptations to facilitate the export of photosynthates from the leaves to the root system. In our study, the observed pattern could not be explained by different light conditions or tree height, as all our experimental trees were of similar size and were growing almost under the same light exposures. Moreover, considering that there is only little genetic variation among A. fargesii populations along an altitudinal gradient (Cheng et al. 2016), it is reasonable to attribute the observed altitudinal trends mainly to individual phenotypic plasticity. More studies are required to draw firm conclusions on this.
4.1 Water relations, tissues non-structural carbohydrate, and morphological traits
We observed there was a decrease in midday leaf water potential with increasing elevation, the midday leaf water potential at a high elevation site (3200 m, −3.70 ± 0.20 MPa) was significantly more negative than the midday leaf water potential obtained at a low elevation site (2600 m, −2.50 ± 0.10 MPa). The observed decline in leaf water potential was consistent with what has been found in a previous study (Gieger and Leuschner 2004). One possibility could be that low soil temperature decreased the water uptake capacity of roots (Wan et al. 2001). The result is reinforced by the fact that leaf δ13C (water use efficiency, Farquhar et al. 1989) was significantly different among altitudes, with the greates values at 3200 m, suggesting that plants show acclimation to drying by freezing and low temperature at subalpine treelines in spite of greater water availability (Li et al. 2004). Hikosaka et al. (2021) showed that leaf N based on the area in Fagus crenata increases at higher altitudes, whereas leaf N did not change with increasing altitude in the present study, indicating that N limitation may not play a key role in the growth of A. fargesii at treeline. In our study, main stem diameter showed an increasing trend with increasing altitudes, but there was a reduction in tree height with increasing altitudes, which might be due to the low temperature that suppressed meristematic growth of primary meristems (Körner 2003). Similarly, previous studies have shown that a windy climate and snow cover caused stunted tree stature at high altitudes (Körner 2003; Körner 2012). Stem growth reduction at the treeline may also reduce transport distance and thus facilitate efficient water and nutrient transport in plants. The decrease in temperature with higher altitudes may contribute to the reduction in specific leaf area. In addition, low specific leaf area contributes to water storage (Ishii et al. 2014) and is often associated with lower NSC concentration (Li et al. 2016). Possibly lower specific leaf area values could allow more flexible storage of carbohydrates and enable trees to better compensate for temporal variations in source and sink strengths because low specific leaf area is linked to enhancement of leaf longevity and thus long-term carbon assimilation (Mediavilla et al. 2008). Dumlao et al. (2012) showed that winter active deciduous species show increases of specific leaf area that go along with a higher capacity for phloem loading when grown under low temperatures.
There is evidence that labile carbohydrate concentrations increase when approaching the treeline (Hoch and Körner 2003), which has been interpreted as a proof of increased growth limitation at high altitudes. However, Sveinbjörnsson (2000) pointed out that labile carbohydrates have multiple functions in plants, and increases in labile carbohydrate concentrations could also be an acclimation to higher risk of late frost. Therefore, evidence of higher labile carbohydrate concentrations at treeline presented by Hoch and Körner (2003) is largely circumstantial. In our study, mean total soluble sugar concentrations across all tissues remain either constant or tended to increase with rising elevation. This indicates that the decrease in height growth is not driven directly by a lack of available carbohydrates. The concentrations of starch in the seedling studied here were similar to results by Li et al. (2013) in Populus yunnanensis, by Yu et al. (2016) in C. lanceolata, and by Simard et al. (2013) in larch and spruce, but were much higher than the results reported by Hoch and Körner (2003). However, the direct comparison of the data by Hoch and Körner (2003) with the data of this study is ambiguous due to different sampling time and the differences in tree age tested. We sampled leaves at the end of the growing season, while Hoch and Körner (2003) sampled during the growing season. Moreover, in our study, the measurement of NSC was sampled from tree seedlings, while in the study by Hoch and Körner (2003) it was sampled from adult trees. It has been reported that carbohydrate concentrations were generally higher in seedlings than those in adult trees (Weber et al. 2018). For example, carbohydrate concentrations in the branches of 2-year-old seedlings are approximately three to four times of those in 100-year-old trees of the same species (Weber et al. 2018). Alternatively, some research identified the lipid concentrations of pines were quite high, which are the prime carbon storage compounds in pines (Hoch et al. 2002; Hoch and Körner 2003). It is possible that the accumulated lipids in the investigated pine species in the study of Hoch and Körner (2003) is responsible for the low NSC concentrations compared to those observed in our study. Additional research would be needed to clarify and quantify this possible explanation.
4.2 Functional areas of phloem and xylem as well as transport conduit diameter
In this study, xylem area fraction declined with increasing altitude, and therefore reduced stem hydraulic efficiency in comparison with those of tree seedlings at low altitude. This reduced hydraulic efficiency reflects that A. fargesii seedlings modify its xylem hydraulic system in response to limited water conditions due to the freeze-induced water shortage (Vaganov et al. 1999) or due to water loss by wind (Mayr et al. 2002). Although the xylem area fraction decreased at high altitude, no corresponding effect on the cell wall thickness was observed, indicating tree seedlings from high elevation have sufficient safety margins and are capable of embolism resistance to cold stresses (Mayr et al. 2003). Previous studies showed an increase in cell wall thickness with elevation, which is probably related to increased mechanical stress from wind and snow loads (Mayr et al. 2006). In our study, the absence of differences in cell wall thickness across elevations indicates that long-term adaptation responses to harsh microclimatic conditions already occur at relative low elevation 2600 m, and that the cell wall thickness may not increase linearly with increasing altitudes as a thick cell wall would require high carbon costs. Moreover, the observed similar lumen diameter was in contrast with other studies showing that xylem cell size decreased with rising altitudes (Gindl et al. 2001; Zimowski et al. 2014; Rossi et al. 2015; Cocozza et al. 2016) because trees from high elevation are expected to be in a more demanding environment (Mayr et al. 2019). Narrow tracheids are presumed to be more resistant to freeze–thaw embolism (Davis et al. 1999). It is estimated that conifers with a mean tracheid lumen diameter wider than 30 μm are vulnerable to freeze–thaw embolism (Pittermann and Sperry 2003). In our study, the main tracheid lumen diameter is within the safe range of tracheid lumen diameter (6–18 μm). Furthermore, a decline in the leaf:sapwood area observed in our study implies trees may adjust the leaf:sapwood area to prevent cavitations rather than by changing the lumen diameter (Martínez-Vilalta et al. 2009).
Our results are largely consistent with our hypothesis that, compared with the low elevation tree seedlings, high elevation seedlings require higher phloem:leaf area because the transport of sugar in the phloem is likely to be slower with increasing altitude. It is well known that the viscosity of sugar solutions increases at low temperatures (Migliori et al. 2007). A four-degree difference in the growing season temperature between the 2600 and 3200 m altitude corresponds approximately to a 15% higher viscosity of a sucrose solution at the same concentration (~20 w/w) as the phloem sap (Telis et al. 2007). This would translate into 15% more phloem needed at 3200 m in comparison to 2600 m altitude, if all other issues concerning phloem transport (e.g., conductance per tube, the pressure difference between sources and sinks) would remain equal. Moreover, in our study, tree seedlings show a decline in height growth, while the phloem:xylem area increases with rising altitude, which may be explained by greater needs for the transport of sugars under cold environments. Similarly, under drought stress environmental conditions, for declining trees, phloem formation has priority over xylem formation for the long-term survival of a tree (Gričar et al. 2009). Our results here support earlier findings that the ratio of annually produced phloem to xylem cell number was related and phloem:xylem area is larger for smaller trees than for larger trees (e.g., Mencuccini et al. 2011; Jyske and Hölttä 2015). In addition, PCA analysis showed that a large xylem area was associated with a high growth rate. This may be due to the fact that the xylem area has a direct association with stem hydraulic conductivity, which supports high gas exchange and hence high growth rates (Meinzer et al. 2010). The non-collapsed phloem area also correlated positively with growth rate, which indicates that an efficient sugar transport may avoid non-structural carbohydrate accumulation and thus permit high gas exchange rates. Interestingly, there were strong relationships between annual growth rate and xylem:phloem area (Fig. 6). This was consistent with the idea that the xylem–phloem ratio is a good indicator of tree vitality (Gričar 2012).
5 Conclusion
Our results demonstrated that the adjustment of the A. fargesii seedlings transport system to elevation occurred primarily through reductions in leaf:sapwood area, and enhancement in xylem:leaf area and phloem:xylem area, but not through differences in vessel lumen diameter or in the sensitivity of leaf physiology to altitude. We suggest that the allocation between leaf area (carbon gain), xylem (water transport), and phloem (carbon transport) could be important indicators in understanding the adaptation of trees at the treeline.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Antikainen J (2017) Wood cellular structure evaluation using image analysis methods. Machine Vision Applications (MVA), 2017 Fifteenth IAPR International Conference. https://doi.org/10.23919/MVA.2017.7986918
Cernusak LA, Arthur DJ, Pate JS, Farquhar GD (2003) Water relations link carbon and oxygen isotope discrimination to phloem sap sugar concentration in Eucalyptus globulus. Plant Physiol 131:1544–1554. https://doi.org/10.1104/pp.102.016303
Cheng X, Lin K, Jiang Y, Yang Z, Huang X (2016) Genetic diversity of Abies georgei var. smithii at different altitudes in jade dragon snow mountain. Acta Agric Univ Jiangxiensis 38:654–659
Cocozza C, Palombo C, Tognetti R, La Porta N, Anichini M, Giovannelli A, Emiliani G (2016) Monitoring intra-annual dynamics of wood formation with microcores and dendrometers in Picea abies at two different altitudes. Tree Physiol 36:832–846. https://doi.org/10.1093/treephys/tpw009
Craig H (1957) Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochim Cosmochim Acta 12:133–149. https://doi.org/10.1016/0016-7037(57)90024-8
Creese C, Benscoter AM, Maherali H (2011) Xylem function and climate adaptation in Pinus. Am J Bot 98:1437–1445. https://doi.org/10.3732/ajb.1100123
Davis SD, Sperry JS, Hacke UG (1999) The relationship between xylem conduit diameter and cavitation caused by freezing. Am J Bot 86:1367–1372. https://doi.org/10.2307/2656919
Dawes MA, Philipson CD, Fonti P, Bebi P, Ttenschwiler SH, Hagedorn F, Rixen C (2015) Soil warming and CO2 enrichment induce biomass shifts in alpine tree line vegetation. Glob Chang Biol 21:2005–2021. https://doi.org/10.1111/gcb.12819
Dumlao MR, Darehshouri A, Cohu CM, Muller O, Mathias J, Adams WW III, Demmig-Adams B (2012) Low temperature acclimation of photosynthetic capacity and leaf morphology in the context of phloem loading type. Photosynth Res 113:181–189. https://doi.org/10.1007/s11120-012-9762-5
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Ann Rev Plant Physiol Mol Biol 40:503–537. https://doi.org/10.1146/annurev.pp.40.060189.002443
Gieger T, Leuschner C (2004) Altitudinal change in needle water relations of Pinus canariensis and possible evidence of a drought-induced alpine timberline on Mt. Teide, Tenerife Flora 199:100–109. https://doi.org/10.1078/0367-2530-00139
Gindl W, Grabner M, Wimmer R (2001) Effects of altitude on tracheid differentiation and lignification of Norway spruce. Can J Bot 79:815–821. https://doi.org/10.1139/b01-060
Gričar J (2012) Cambial cell production and structure of xylem and phloem as an indicator of tree vitality: a review. IntechOpen. https://doi.org/10.5772/29944
Gričar J, Krže L, Čufar K (2009) Number of cells in xylem, phloem and dormant cambium in silver fir (Abies alba), in trees of different vitality. IAWA J 30:121–133. https://doi.org/10.1163/22941932-90000208
Hankin LE, Higuera PE, Davis KT, Dobrowski SZ (2018) Accuracy of node and bud-scar counts for aging two dominant conifers in western North America. Forest Ecol Manag 427:365–371. https://doi.org/10.1016/j.foreco.2018.06.001
Hikosaka K, Kurokawa H, Arai T, Takayanagi S, Tanaka HO, Nagano S, Nakashizuka T, Satake A (2021) Intraspecific variations in leaf traits, productivity and resource use efficiencies in the dominant species of subalpine evergreen coniferous and deciduous broad-leaved forests along the altitudinal gradient. J Ecol 109:1804–1818. https://doi.org/10.1111/1365-2745.13603
Hoch G, Körner C (2003) The carbon charging of pines at the climatic treeline: a global comparison. Oecologia 135:10–21. https://doi.org/10.1007/s00442-002-1154-7
Hoch G, Popp M, Köner C (2002) Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos 98:361–374. https://doi.org/10.1034/j.1600-0706.2002.980301.x
Hölttä T, Vesala T, Sevanto S, Perämäki M, Nikinmaa E (2006) Modeling xylem and phloem water flows in trees according to cohesion theory and Münch hypothesis. Trees Struct Funct 20:67–78. https://doi.org/10.1007/s00468-005-0014-6
Hölttä T, Mencuccini M, Nikinmaa E (2009) Linking phloem function to structure: analysis with a coupled xylem-phloem transport model. J Theor Biol 259:325–337. https://doi.org/10.1016/j.jtbi.2009.03.039
Hubick KT, Farquhar GD, Shorter R (1986) Correlation between water-use efficiency and carbon isotope discrimination in diverse peanut (Arachis) germplasm. Aust. J. Plant Physiol 13:803–816. https://doi.org/10.1071/PP9860803
Ishii HR, Azuma W, Kuroda K, Sillett SC (2014) Pushing the limits to tree height: could foliar water storage compensate for hydraulic constraints in Sequoia sempervirens? Funct Ecol 28:1087–1093. https://doi.org/10.1111/1365-2435.12284
Jyske T, Hölttä T (2015) Comparison of phloem and xylem hydraulic architecture in Picea abies stems. New Phytol 205:102–115. https://doi.org/10.1111/nph.12973
Körner C (1998) A re-assessment of high elevation treeline positions and their explanation. Oecologia 115:445–459. https://doi.org/10.1007/s004420050540
Körner C (2003) Alpine plant life: functional plant ecology of high mountain ecosystems. Springer, Berlin-Heidelberg
Körner C (2012) Alpine treelines. Springer, Basel
Li CY, Liu SR, Berninger F (2004) Picea seedlings show apparent acclimation to drought with increasing altitude in the eastern Himalaya. Trees 18:277–283. https://doi.org/10.1007/s00468-003-0304-9
Li L, Zhang YB, Luo JX, Korpelainen H, Li CY (2013) Sex-specific responses of Populus yunnanensis exposed to elevated CO2 and salinity. Physiol Plant 147:477–488. https://doi.org/10.1111/j.1399-3054.2012.01676.x
Li N, He NP, Yu GR, Wang QF, Sun J (2016) Leaf non-structural carbohydrates regulated by plant functional groups and climate: evidences from a tropical to cold-temperate forest transect. Ecol Indic 62:22–31. https://doi.org/10.1016/j.ecolind.2015.11.017
Losso A, Anfodillo T, Ganthaler A, Kofler W, Markl Y, Nardini A, Oberhuber W, Purin G, Mayr S (2018) Robustness of xylem properties in conifers: analyses of tracheid and pit dimensions along elevational transects. Tree Physiol 38:212–222. https://doi.org/10.1093/treephys/tpx168
Martínez-Vilalta J, Cochard H, Mencuccini M, Sterck F, Herrero A, Korhonen JFJ, Llorens P, Nikinmaa E, Nolè A, Poyatos R, Ripullone F, Sass-Klaassen U, Zweifel R (2009) Hydraulic adjustment of scots pine across Europe. New Phytol 184:353–364. https://doi.org/10.1111/j.1469-8137.2009.02954.x
Mayr S, Wolfschwenger M, Bauer H (2002) Winter-drought induced embolism in Norway spruce (Picea abies) at the Alpine timberline. Physiol Plant 115:74–80. https://doi.org/10.1034/j.1399-3054.2002.1150108.x
Mayr S, Schwienbacher F, Bauer H (2003) Winter at the alpine timberline. Why does embolism occur in Norway spruce but not in stone pine? Plant Physiol 131:780–792. https://doi.org/10.1104/pp.011452
Mayr S, Hacke U, Schmid P, Schwienbacher F, Gruber A (2006) Frost drought in conifers at the alpine timberline: xylem dysfunction and adaptations. Ecology 87:3175–3185. https://doi.org/10.1890/0012-9658(2006)87[3175:FDICAT]2.0.CO;2
Mayr S, Cochard H, Ameglio T, Kikuta SB (2007) Embolism formation during freezing in the wood of Picea abies. Plant Physiol 143:60–67. https://doi.org/10.1104/pp.106.085704
Mayr S, Schmid P, Beikircher B, Feng F, Badel E (2019) Die hard: timberline conifers survive annual winter embolism. New Phytol 226:13–20. https://doi.org/10.1111/nph.16304
McNown RW, Sullivan PF (2013) Low photosynthesis of treeline white spruce is associated with limited soil nitrogen availability in the Western Brooks Range, Alaska. Funct Ecol 27:672–683. https://doi.org/10.1111/1365-2435.12082
Mediavilla S, Garcia-Cuidad A, Garcia-Criado B, Escudero A (2008) Testing the correlations between leaf life span and leaf structural reinforcement in 13 species of European Mediterranean woody plants. Funct Ecol 22:787–793. https://doi.org/10.1111/j.1365-2435.2008.01453.x
Meinzer FC, McCulloh KA, Lachenbruch B, Woodruff DR, Johnson DM (2010) The blind men and the elephant: the impact of context and scale in evaluating conflicts between plant hydraulic safety and efficiency. Oecologia 164:287–296. https://doi.org/10.1007/s00442-010-1734-x
Mencuccini M, Hölttä T, Martinez-Vilalta J (2011) Comparative criteria for models of the vascular transport systems of tall trees. In: Meinzer FC, Lachenbruch B, Dawson T (eds) Size-and age-related changes in tree structure and function. Springer, Dordrecht, pp 309–339
Migliori M, Gabriele D, Sanzo RD, Cindio BD, Correra S (2007) Viscosity of multicomponent solutions of simple and complex sugars in water. J Chem Eng Data 52:1347–1353. https://doi.org/10.1021/je700062x
Mitchell AK (1998) Acclimation of Pacific yew (Taxus brevifolia) foliage to sun and shade. Tree Physiol 18:749–757
Münch E (1930) Die stoffbewegungen in der Pflanze. Fischer, Jena, p 234
Osaki M, Shinano T, Tadano T (1991) Redistribution of carbon and nitrogen compounds from the shoot to the harvesting organs during maturation in field crops. Soil Sci Plant Nutr 37:117–128. https://doi.org/10.1080/00380768.1991.10415017
Pittermann J, Sperry J (2003) Tracheid diameter is the key trait determining the extent of freezing-induced embolism in conifers. Tree Physiol 23:907–914. https://doi.org/10.1093/treephys/23.13.907
Poorter L, Mcdonald I, Alarcón A, Fichtler E, Licona JC, Peña-Claros M, Sterch F, Villegas Z, Sass-Klaassen U (2010) The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol 185:481–492. https://doi.org/10.1111/j.1469-8137.2009.03092.x
Pritzkow C, Heinrich I, Grudd H, Helle G (2014) Relationship between wood anatomy, tree-ring widths and wood density of Pinus sylvestris L. and climate at high latitudes in northern Sweden. Dendrochronologia 32:295–302. https://doi.org/10.1016/j.dendro.2014.07.003
Rossi S, Deslauriers A, Griçar J, Seo JW, Rathgeber CB, Anfodillo T, Jalkanen R (2008) Critical temperatures for xylogenesis in conifers of cold climates. Glob Ecol Biogeogr 17:696–707. https://doi.org/10.1111/j.1466-8238.2008.00417.x
Rossi S, Cairo E, Krause C, Deslauriers A (2015) Growth and basic wood properties of black spruce along an alti-latitudinal gradient in Quebec. Canada Ann Forest Sci 72:77–87. https://doi.org/10.1007/s13595-014-0399-8
Růžička K, Ursache R, Hejátko J, Helariutta Y (2015) Xylem development-from the cradle to the grave. New Phytol 207:519–535. https://doi.org/10.1111/nph.13383
Savage JA, Clearwater MJ, Haines DF, Klein T, Mencuccini M, Sevanto S, Turgeon R, Zhang C (2016) Allocation, stress tolerance and carbon transport in plants: how does phloem physiology affect plant ecology? Plant Cell Environ 39:709–725. https://doi.org/10.1111/pce.12602
Sevanto S, Ryan M, Turin Dickman L, Derome D, Patera A, Defraeye T, Pangle RE, Hudson PJ, Pockman WT (2018) Is desiccation tolerance and avoidance reflected in xylem and phloem anatomy of two co-existing arid-zone coniferous trees? Plant Cell Environ 27:672–683. https://doi.org/10.1111/1365-2435.12082
Simard S, Giovannelli A, Treydte K, Traversi ML, King GM, Frank D, Fonti P (2013) Intra-annual dynamics of non-structural carbohydrates in the cambium of mature conifer trees reflects radial growth demands. Tree Physiol 33:913–923. https://doi.org/10.1093/treephys/tpt075
Steppe K, Vandegehuchte MW, Tognetti R, Mencuccini M (2015) Sap flow as a key trait in the understanding of plant hydraulic functioning. Tree Physiol 35:341–345. https://doi.org/10.1093/treephys/tpv033
Susiluoto S, Hilasvuori E, Berninger F (2010) Testing the growth limitation hypothesis for subarctic scots pine. J Ecol 98:1186–1195. https://doi.org/10.1111/j.1365-2745.2010.01684.x
Sveinbjörnsson B (2000) North American and European treelines: external forces and internal processes controlling position. Ambio 29:388–395
Telis VRN, Telis-Romero J, Mazzotti HB, Gabas AL (2007) Viscosity of aqueous carbohydrate solutions at different temperatures and concentrations. Int J Food Prop 10:185–195. https://doi.org/10.1080/10942910600673636
Ter Braak CJF, Smilauer P (2002) Canoco for windows version 4.5. Centre for Biometry Wageningen, Wagenigen
Vaganov EA, Hughes MK, Kirdyanov AV, Schweingruber FH, Silkin PP (1999) Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 400:149–151
Wan XC, Zwiazek JJ, Lieffers VJ, Landhäusser SM (2001) Hydraulic conductance in aspen (Populus tremuloides) seedlings exposed to low root temperatures. Tree Physiol 21:691–696. https://doi.org/10.1093/treephys/21.10.691
Wang KY (2004) Processes of subalpine Forest ecosystem in the west of Sichuan. Sichuan Science and Technology Press, Chengdu
Weber R, Schwendener A, Schmid S, Lambert S, Wiley E, Landhäusser SM, Hartmann H, Hoch G (2018) Living on next to nothing: tree seedlings can survive weeks with very low carbohydrate concentrations. New Phytol 281:107–118. https://doi.org/10.1111/nph.14987
Wiley E, Helliker B (2012) A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol 195:285–289. https://doi.org/10.1111/j.1469-8137.2012.04180.x
Yu L, Dong TF, Lu YB, Song MY, Duan BL (2016) Ecophysiological responses of Cunninghamia lanceolata to nongrowing-season warming, nitrogen deposition, and their combination. Photosynthetica 54:598–610. https://doi.org/10.1007/s11099-016-0647-2
Zhang L, Copini P, Weemstra M, Sterck FJ (2016) Functional ratios among leaf, xylem and phloem areas in branches change with shade tolerance, but not with local light conditions, across temperate tree species. New Phytol 209:1566–1575. https://doi.org/10.1111/nph.13731
Zhang L, Chen Y, Hao G, Ma K, Bongers F, Sterck FJ (2020) Conifer and broadleaved trees differ in branch allometry but maintain similar functional balances. Tree Physiol 40:511–519. https://doi.org/10.1093/treephys/tpz139
Zimowski M, Leuschner HH, Gärtner H, Bergmeier E (2014) Age and diversity of Mediterranean dwarf shrublands: a dendrochronological approach along an altitudinal gradient on Crete. J Veg Sci 25:122–134. https://doi.org/10.1111/jvs.12067
Acknowledgements
The research was supported by the National Science Foundation of China (No. 31770439). T. Jyske was supported by post-doctoral research grants from the Academy of Finland (no. 250299) and JSPS KAKENHI (no. 26·04395). Sílvia Roig Juan is thanked for her skillful technical assistance.
Funding
The research was supported by the National Science Foundation of China (No. 31770439). T. Jyske was supported by post-doctoral research grants from the Academy of Finland (no. 250299) and JSPS KAKENHI (no. 26•04395).
Author information
Authors and Affiliations
Contributions
Yuanbin Zhang led the manuscript preparation with substantial contributions from all the coauthors. Tuula Jyske, Jukka Antikainen, Teemu Hölttä, Qiao Gao, and Frank Berninger contributed substantially to the data collection and also to the critical revisions of the manuscript. Baoli Duan supervised the research work and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Maurizio Mencuccini
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Jyske, T., Pumpanen, J. et al. Adaptation of Abies fargesii var. faxoniana (Rehder et E.H. Wilson) Tang S Liu seedlings to high altitude in a subalpine forest in southwestern China with special reference to phloem and xylem traits. Annals of Forest Science 78, 85 (2021). https://doi.org/10.1007/s13595-021-01095-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-021-01095-8