Abstract
An ethanol extract of Lasius fuliginosus ant nests was found to be highly effective against nosemosis in apiary honey bees. Two concentrations of the extract (300 mg and 1500 mg/100 ml sugar syrup) were tested on honey bees infected by nosemosis, and both significantly reduced the number of Vairimorpha ceranae DNA copies. Administration of the extract also had a positive effect on the honey bees’ immune system, increasing the expression of genes encoding immune peptides (abaecin, defensin-1, hymenoptaecin). The extract was subjected to detailed biochemical analysis for the presence of total carbohydrates (22.1 µg/ml), proteins (26.7 µg/ml), and phenolic compounds (63.8 μM) and also to assess its reducing activity (69.2% for DPPH radicals and 94% for the ABTS method). FTIR analysis revealed a number of groups and types of organic compounds (including fatty acids, triglycerides, formic acid, and phenols), the presence of which may have a decisive influence on the preparation’s high level of biological activity.
Similar content being viewed by others
1 Introduction
The associations between ants on the one hand and fungal and bacterial microorganisms on the other are well known to science (e.g., Bailey 1920; Currie 2001; Mueller et al. 2001; Boomsma et al. 2005; Goes et al. 2020). One of the best-known examples of the symbiosis between ants and fungi is the cultivation of Basidiomycota by fungus-growing ants (Attini) for the provision of food (Mueller et al. 1998). It turns out, however, that the functioning of this association is more complex and involves additional elements, such as fungi parasitizing the cultivar or symbiotic bacteria producing substances protecting the cultivation from infection. The whole system is an excellent example of coevolution (Currie et al. 1999; Currie and Stuart 2001). But the equilibrium of this system is controlled by ants, specifically by their symbionts. These were illustrated inter alia by Sen et al. (2009), who isolated from the insects and their nests strains of Pseudonocardia and Amycolatopsis bacteria, the activity of which is distinctly antifungal.
It transpires that the temperate-climate jet black ant Lasius fuliginosus is also an extremely interesting subject of study, with regard not only to its biology but also to the presence of highly active biological substances in both the ant itself and its nest. The jet black ant usually establishes its nests in the hollows of old trees growing in forests, parks, or singly in open landscapes, across practically the entire Palearctic. The carton, which is the basic building material for the nests, consists of wood, comminuted into fragments by worker ants, mineral particles, the ants’ own saliva, and aphid honeydew. Its total volume inside a single tree can be as much as 150 dm3. The honeydew provides sustenance for symbiotic Ascomycota fungi, the dense mycelium network of which penetrates, reinforces, and stabilizes the carton structure (Seifert 2018; Maschwitz and Hölldobler 1970).
More and more is becoming known about the interrelationships between jet black ants and other organisms. Microbiological analysis of the heads of these ants has revealed the presence of a strain of the actinomycete Streptomyces lasiicapitis, a taxon new to science. These bacteria produce kanchanamycin, a substance with antifungal activity (Ye et al. 2017). These data on the presence of actinomycetes in ants concur with those given by Kaczmarczyk-Ziemba et al. (2020), who discovered bacteria belonging, inter alia, to Streptomycetaceae in both the ants themselves and their nest material.
Krishanti et al. (2018) carried out a similar study on termite nests. From their discovery of a series of actinomycete strains that produce antimicrobial substances, they inferred that termite nests are a new source of bactericidal substances with a very considerable therapeutic potential.
Brinker et al. (2019) carried out an interesting two-year study of the fungi and bacteria in the nest carton of L. fuliginosus and the surrounding soil. They found the nest’s mycoflora to be highly stable and that it resembled the assemblages of fungi in the nest’s surroundings, despite the simultaneous fluctuations in the structures of the bacterial assemblages and the differences between those of the nest and the surroundings. Those authors also demonstrated that preparations made from ants exhibited an inhibitory effect on the pathogenic fungus Metarhizium sp. In contrast, they found that an extract of ants incubated together with nest fungi did not display any inhibitory effect whatsoever; quite the reverse, in fact: mycelium growth accelerated in comparison with the control sample. This testifies to the long-term coevolution of an active mechanism by which L. fuliginosus manages, as it were, the assemblages of microorganisms, this process probably being mediated by the host’s secretions.
The carton nests of the jet black ant evidently have a very considerable research potential, as further studies have shown. Wagner et al. (2021) demonstrated the considerable biological activity of an aqueous extract of the nest carton of these ants, specifically, its antiproliferation potential with respect to human melanoma cells. Kunat et al. (2020) likewise examined the properties of an aqueous extract of carton, but for treating nosemosis in the honey bee, and found it to have antifungal activity. Due to its activity, the preparation is under legal protection in the Patent Office of the Republic of Poland (No. Pat. 241494).
Nosemosis is a disease of honey bees caused by fungi of the genus Vairimorpha. These are obligate, intracellular parasites colonizing the mid-gut of honey bees. The life cycle of Vairimorpha spp. begins when the parasite enters the bee via the oral route. The infection results in physiological and anatomical changes in the intestinal cells, leading to digestive disorders and malnutrition in honey bees (Ptaszyńska et al. 2014, 2016). In the genus Vairimorpha, V. ceranae is the dominant species in honeybee infections in Canada, but also in many other countries of the world (Emsen et al. 2016). It can occur alone or together with V. apis. Both pathogens are a serious problem for beekeeping, because they lead to reduced lifespan of infected bees and increased winter mortality. The negative influence of nosemosis infection on adult honey bee population size and brood production also causes a decrease in honey production (Botias et al. 2013). At the present time, there are no legally approved compounds for effectively combatting nosemosis (Regulation 2377/90, European Commission 2009). There is thus an urgent need to search for other ways of treating nosemosis.
In light of this, we decided to examine the activity of an ethanol extract obtained from jet black ant nests. The method of obtaining and using the extract has the status of an application to the Patent Office of the Republic of Poland (No. P441848). This paper presents the results of our study.
2 Material and methods
2.1 Field collection and preliminary laboratory preparation of research material
The research material, consisting of fragments of the carton nests of the jet black ant L. fuliginosus, was collected from a hollow in an oak tree (Quercus robur L.) growing in the Pieszowola manor park in the buffer zone of the Polesie National Park in Poland (GPS, N 51°29′39.7″ E 023°10′13.1″) (Figure 1A). About 2 l of this material (Figure 1B, C) was obtained and placed in a sterile vessel. In the laboratory, the carton was cleared of artifacts and ants under an Olympus SZ51 stereoscopic microscope and then deep-frozen (− 80 °C).
2.2 Preparation of the ethanol extract
A 1:10 weight-to-volume ratio of ant nest material and 96% ethanol was first stirred, then homogenized in a blade homogenizer. Thereafter, the preparation was sonicated three times with breaks for cooling in ice (80% amplitude, 4-min cycle–30 s pulse/30 s). In the next stage, the preparation was extracted on a mechanical cradle over a period of 5 days, filtered through a Miracloth filter, and then centrifuged (10,000 × g for 15 min at 4 °C). The solvent was removed from the preparation by desiccation in a vacuum centrifuge. The solvent-free sludge obtained was used in the further research.
2.3 Biochemical characteristics of the extract
2.3.1 Carbohydrates, proteins, and phenolic compounds
These were determined according to the procedure described in Wagner et al. (2021). The total carbohydrate content of the ethanol extract was determined by phenol–sulphuric acid assay with D-glucose as standard (DuBois et al. 1956). Protein concentrations were determined using Bradford reagent and bovine serum albumin as standard (Bradford 1976). The total content of phenolic compounds was determined with diazosulphanilamide using the DASA test (Malarczyk 1989), where the absorbance was measured at 500 nm and vanillic acid was the standard (Wagner et al. 2021).
Assay of the relative level of superoxide anion radicals (SORs)
The relative level of SORs was estimated spectrophotometrically based on the detection of the superoxide-induced formation of formazan from nitrotetrazolium blue (NBT) under alkaline conditions (Jaszek et al. 2006). The reaction mixture containing 3 ml of distilled water, 0.05 ml of 1 M NaOH, 0.1 ml of 5 mM NBT solution, and 0.1 ml of the sample was incubated (30 min at 20 °C); the absorbance was measured at 560 nm.
2.4 Determination of the antioxidative potential
2.4.1 Free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH)-scavenging test
The total antioxidant capacity of the ethanol extract was determined using the DPPH radical as a reagent, according to the procedure described by Paduch et al. (2008). This method is based on the ability of 1,1-diphenyl-2-picrylhydrazyl (DPPH) to decolourize in the presence of antioxidants.
[2,2’-Azinobis-(3-ethylbenzothiazoline-6-sulphonic acid)] (ABTS) radical-scavenging test
The ABTS radical-scavenging activity of the ethanol extract was determined using the methods of van den Berg et al. (1999), Duo-Chuan (2006), and Re et al. (1992) with modifications. Briefly, a stock solution of 7.4 mM ABTS and 2.6 mM potassium persulfate was stabilized for 16 h at room temperature before use, then the mixture was diluted with PBS buffer to 0.7 absorbance units at 734 nm. Ten microliter of the test sample was added to 990 µl of the ABTS radical solution. The rate of change in free radical scavenging was measured at 734 nm relative to water.
2.5 FTIR (Fourier-transform infrared spectroscopy) analysis
The FTIR-ATR spectra were obtained on a Vertex 70 spectrometer coupled with a Hyperion 3000 microscope using an ATR-germanium crystal (Bruker, USA) (resolution 4 cm−1, 64 scans per spectrum). The samples had been previously desiccated at 37 °C for 24 h to remove all unbound water. Spectra were analyzed using OPUS 7.0 software (Bruker, USA).
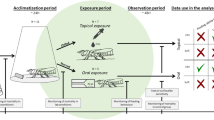
2.6 Preparation of the apiary experiment
The brood frame from a hive infected with Vairimorpha ceranae, taken from a single colony from an apiary situated at Łuszczów Drugi in Lublin Province in Poland (51°17′36″N 22°45′13″E), was placed in an incubator at 34.5 °C and 85% relative humidity. After emergence, the 1-day-old honey bees Apis meliffera meliffera were placed in 4 cages (50 honey bees per cage) and marked with a special marker (Figure 2A).
The honey bees were divided into 3 experimental groups:
-
Control group—honey bees fed only with sugar syrup for 3 days;
-
Test group I (10A, 28A)—honey bees fed with sugar syrup and sprayed twice a day with the extract in a concentration of 300 mg/100 ml of sugar syrup for 3 days;
-
Test group II (10B, 28B)—honey bees fed with sugar syrup and sprayed twice a day with the extract in a concentration of 1500 mg/100 ml of sugar syrup for 3 days.
After 3 days, the honey bees were returned to the parent colony from which they were taken in the apiary (Figure 2B). On days 10 and 28 of the experiment, the marked honey bees were collected for further analysis in order to determine the number of Vairimorpha ceranae DNA copies and the expression level of the immune peptide-encoding genes abaecin, defensin-1 and, hymenoptaecin using real-time PCR.
2.7 DNA extraction
DNA was extracted from the abdomens of honeybees from the control and both test groups. Three abdomens from each group were placed in Eppendorf tubes. The lysis buffer and proteinase K were then added to digest the proteins in the test samples. The samples thus prepared were incubated at 56 °C for 24 h. After incubation, DNA was isolated in accordance with the manufacturer’s protocol (Dneasy Blood & Tissue Kits, QIAGEN). The isolated DNA samples were stored in a freezer at − 20 °C. The presence of Vairimorpha ceranae was confirmed by duplex PCR (Martin-Hernandez et al. 2007).
2.8 Determination of the degree of infection with Vairimorpha ceranae
The degree of infection with Vairimorpha ceranae in the control and test samples was determined using real-time PCR with the commercially available MycoReal Kit (Ingenetix, Vienna, Austria). Five microliter of DNA, whose concentration ranged from 1 to 1.5 µg was added to the reaction mixture. The real-time PCR conditions were: 50 °C—2 min, 95 °C—20 s, 45 cycles (95 °C—5 s, 60 °C—1 min). The results were read off the standard curve plotted on the basis of PCR amplification performed with several dilutions of the standardized DNA sample containing 10,000 copies of Vairimorpha ceranae/µl (MycoReal Kit, Ingenetix).
2.9 RNA isolation, reverse transcription, and gene expression
RNA was isolated from 3 abdomens of honey bees from each of the control and the two test groups using the commercially available Universal Purification Kit (EURx, Gdańsk, Poland). The isolated RNA was purified from residual DNA using the commercially available TURBO DNA-free™ Kit (ThermoFischer Scientific, Waltham, MA, USA). The RNA concentration was determined in the purified material (absorbance measurement at λ = 260 nm) and the purity of the RNA obtained was assessed (measurement ratio at two wavelengths 260/280 nm). RNA samples (1 µg) were reverse-transcribed using the High-Capacity cDNA Reverse Transcription Kit with an Rnase inhibitor (ThermoFischer Scientific, Waltham, MA, USA). The primers for the individual genes, i.e., abaecin, defensin-1, and hymenoptaecin, were used to amplify the cDNA by PCR with real-time product quantity analysis using the StepOnePlusTMReal-Time PCR system the Power SYBR Green PCR Master Mix (Applied Biosystems™, Foster City, CA, USA). The real-time PCR was performed under the following conditions: 95 °C—10 min, 44 cycles (95 °C—15 s, 60 °C—1 min). The relative expression was normalized to reference gene β-actin whose level was constant in all the conditions tested. The relative gene expression was calculated taking into account the reaction efficiency (which was above 90% for all primers) according to the Pfaffl equation (Pfaffl 2001). The primer sequences are shown in Table I.
2.10 Statistical analysis
A repeated measures ANOVA was performed to compare the effect of different concentrations of extracts and duration of infection on selected parameters. Bonferroni adjustment for multiple comparisons was applied. The data were analyzed using TIBCO Statistica 13.3. The experiments were performed in 3 replicates. The results are presented as means with standard deviation.
3 Results
3.1 Biochemical characteristics of the extract
The analysis of the biochemical properties of the ethanol extract obtained from the ant nests yielded total carbohydrates (22.1 µg/ml), proteins (26.7 µg/ml), and phenolic compounds (63.8 µM). The relative SOR level was also determined (Table II). The analysis further demonstrated the strong reducing activity of the ethanol extract (69.2% with the DPPH radicals and 94% with ABTS). The radical-scavenging capacity of the extract is illustrated in Table III.
3.2 FTIR analysis of the extract
Multiple broad peaks were present in the FTIR spectrum of the ethanol extracts (Figure 3). This type of spectrum is typical of complex organic extracts. It showed bands characteristic of fatty acids: 2924 cm−1 (asymmetric stretching of –CH3 and –CH2 groups), 2854 cm−1 (symmetric stretching of –CH3 and –CH2 groups), 1729 cm−1 (stretching vibrations of C = O bands in carboxylic groups), and 1456 cm−1 (bending of –CH3 groups or C = O bands in carboxylic groups). In addition, bands characteristic of glycerine were present in the spectrum: 1418 cm−1 (bending vibrations of –CH2 groups), 1328 cm−1 (bending vibrations of –CH2 groups), and a strong band at 1034 cm−1 (stretching vibrations of C-O groups) (Ahmed et al. 2010). This suggests the presence of triglycerides. The broad band in the 3500–3100 cm−1 region with a peak at 3336 cm−1 (O–H stretching vibrations) could also be associated with glycerine in triglycerides. Lipid substances are likely to be found in ethanol ant nest extracts: it has been reported that ant bodies at different developmental stages are rich in lipids (Bhulaidok et al. 2010; Sihamala et al. 2010). The presence of bands at 1729 cm−1 (stretching vibrations of C = O bands in carboxylic groups) and 1456 cm−1 (bending vibrations of –CH3 groups or C = O bands in carboxylic groups) may also suggest the presence of formic acid, which is likely to appear in extracts of ant nest material and is ethanol-soluble. The spectrum also showed bands at 1596 cm−1 (medium-strength) and 1507 cm−1 (weak-to-medium) (C = C vibrations in the vanillin aromatic ring), 1373 cm−1 (C-O stretching vibrations in phenols), and 1232 cm−1 (C–OH vibrations in syringic acid) (Balachandran and Parimala 2012; Singh and Kumar 2018). These bands may suggest the presence of lignin-derived phenolic compounds; the weak band at 827–827 cm–1 (C-H bending vibrations in p-disubstituted aromatic rings) in the test samples may also provide confirmation of the presence of these compounds. The data on the presence of lignin-derived phenols in the ethanol ant nest extracts tally with the results published by Kristiansen and Amelung (2001). The small band at 895 cm−1 indicates the probable presence of the β-glycosidic bond detected in cellulose chains (Liu and Kim 2017; Szymanska-Chargot and Zdunek 2013), reflecting the partial solubility of cellulose in ethanol (Naz et al. 2016), small quantities of which may have been extracted from the nest material.
3.3 Results of the experiments with honey bees
3.3.1 The degree of infection with Vairimorpha ceranae
The DNA copy number of Vairimorpha ceranae was determined by real-time PCR according to the manufacturer’s protocol.
A repeated measures ANOVA was performed to compare the effect of different concentrations of extracts and duration of infection on the DNA copy number of Vairimorpha ceranae. The test showed that there was a statistically significant difference between timepoints (10th and 28th day), (F(1, 6) = 1007.08, p < 0.001) and interaction between duration of infection and extract concentration (F(2, 6) = 480.18, p < 0.001). The mean values between the fraction-treated honey bees and the 10-day control group pairwise comparisons with Bonferroni adjustment found the DNA copy number to be significantly different between 10B and the control (p < 0.001, 95% C.I. = [6245.38;11,497.47]). There was no statistically significant difference in the mean results between 10A and the control (p = 0.79), but there was such a difference between 10A and 10B (p < 0.001) (Figure 4).
DNA copy number of Vairimorpha ceranae in 10-day-old honey bees. The asterisks denote statistically significant differences ***p < 0.001 (ANOVA) between the 10-day control group and the experimental groups—10A (10-day-old honeybees to which 300 mg of the preparation per 100 ml of sugar syrup were administered) and 10B (10-day-old honeybees to which 1500 mg of the preparation per 100 ml of sugar syrup were administered)
In addition, there were statistically significant differences in the DNA copy number of Vairimorpha ceranae on day 28 between the control and tested concentrations A (p < 0.001, 95% C.I. = [16551.70; 19,086.40]) and B (p < 0.001, 95% C.I. = [13089.70; 15,624.50]). Pairwise comparison showed statistically significant differences between the control and the two concentrations as well as between the two concentrations themselves (p < 0.001) (Figure 5).
DNA copy number of Vairimorpha ceranae in 28-day-old honey bees. The asterisks denote statistically significant differences ***p < 0.001 (ANOVA) between the 28-day control group and the experimental groups—28A (28-day-old honeybees to which 300 mg of the preparation per 100 ml of sugar syrup were administered) and 28B (28-day-old honeybees to which 1500 mg of the preparation per 100 ml of sugar syrup were administered)
Expression level of the genes encoding immune peptides
The expression of the abaecin gene revealed statistically significant differences between timepoints (F(1, 6) = 2160.98, p < 0.001) and interaction between duration of infection and extract concentration (F(2, 6) = 5825.40, p < 0.001). Multiple comparison highlighted significant differences both between formulations 10A (p = 0.001 95% C.I. = [0.421; 1.088]) and 10B (p < 0.001 95% C.I. = [6,539; 5.873]), and the control, as well as between the two concentrations themselves (p ≤ 0.001) (Figure 6). The same applied to the experiment after 28 days, the level of expression of abaecin was more than twice as high as that of the control in the case of 28A, (p = 0.005 95% C.I. = [0.826; 0.196], and 2.7 times higher in the case of 28B (p < 0.001, 95% C.I. = [ 2.114; 1.484] (Fig. 7).
Expression level of the gene encoding the defense peptide abaecin in the total RNA of bee abdomens determined by quantitative RT-PCR. The results are normalized to β-actin. The error bars represent the standard deviation. The asterisks denote statistically significant differences **p < 0.01 and ***p < 0.001 (ANOVA) between the control group and the experimental groups—10A (10-day-old honeybees to which 300 mg of the preparation per 100 ml of sugar syrup were administered), 10B (10-day-old honeybees to which 1500 mg of the preparation per 100 ml of sugar syrup were administered), 28A (28-day-old honeybees to which 300 mg of the preparation per 100 ml of sugar syrup were administered), and 28B (28-day-old honeybees to which 1500 mg of the preparation per 100 ml of sugar syrup were administered). Different lowercase letters indicate statistically significant differences between samples (p ≤ 0.05, ANOVA)
Expression level of the gene encoding the defense peptide defensin in the total RNA of bee abdomens determined by quantitative RT-PCR. The results are normalized to β-actin. The error bars represent the standard deviation. The asterisks denote statistically significant differences **p < 0.01 and ***p < 0.001 (ANOVA) between the control group and the experimental groups—10A (10-day-old honeybees to which 300 mg of the preparation per 100 ml of sugar syrup were administered), 10B (10-day-old honeybees to which 1500 mg of the preparation per 100 ml of sugar syrup were administered), 28A (28-day-old honeybees to which 300 mg of the preparation per 100 ml of sugar syrup were administered), and 28B (28-day-old honeybees to which 1500 mg of the preparation per 100 ml of sugar syrup were administered). Different lowercase letters indicate statistically significant differences between samples (p ≤ 0.05, ANOVA)
Statistically significant differences also occurred for the level of defensin-1 expression between timepoints (F(1, 6) = 683,42, p < 0.001) and the interaction between the duration of infection and extract concentration (F(2, 6) = 230,57, p < 0.001). On the 10th day, both 10A and 10B showed statistically significant differences of 0.07 (0.01), (p = 0.001, 95% CI = [0,545; 1.324]) and 1.5 (0.16), respectively (p = 0.01 95% C.I. = [0.947; 0.168]) in relation to control 1 (0.19) (Figure 8). Similarly, on the 28th day of the experiment, a significant increase in the defensin-1 level was found in preparations 28A (7.3 (1.43)) 28B (16.3 (0.3)) p < 0.001, (95% C.I. = [8.59; 4.02] and [17.58; 13.01], respectively) in relation to control 1(0.19) (Figure 7).
Expression level of the gene encoding the defense peptide hymenoptaecin in the total RNA of bee abdomens determined by quantitative RT-PCR. The results are normalized to β-actin. The error bars represent the standard deviation. The asterisks denote statistically significant differences **p < 0.01 (ANOVA) between the control group and the experimental groups—10A (10-day-old honeybees to which 300 mg of the preparation per 100 ml of sugar syrup were administered), 10B (10-day-old honeybees to which 1500 mg of the preparation per 100 ml of sugar syrup were administered), 28A (28-day-old honeybees to which 300 mg of the preparation per 100 ml of sugar syrup were administered), and 28B (28-day-old honeybees to which 1500 mg of the preparation per 100 ml of sugar syrup were administered). Different lowercase letters indicate statistically significant differences between samples (p ≤ 0.05, ANOVA)
A statistically significant difference between timepoints (F(1, 6) = 7.106, p < 0.04) in the levels of hymenoptaecin expression and no statistically significant interaction between duration of infection and extract concentration was observed. An increase in the level of hymenoptaecin expression was demonstrated by formulation 10B compared to the control (p = 0.008, 95% C.I. [4.540; 0.880]). The same applied to preparation 28B p < 0.001 (95% C.I. = [6.170; 2.850]) (Figure 8).
4 Discussion
The honey bee is the principal pollinator of both cultivated and wild plants. At the global scale, therefore, the importance of this species in the environment and for agriculture cannot be stressed enough (e.g., Calderone 2012; Paudel et al. 2015; Sidhu and Joshi 2016). The consequences of the ongoing dramatic decline in the numbers of bee families, deriving from numerous biotic and abiotic factors (Ptaszyńska 2022), are having a detrimental effect on the ecology and diversity of wild plants, not to mention the yields of cultivated plants (Potts et al. 2016; Stanley et al. 2020).
Diseases are a serious problem for honey bees, among which is nosemosis, caused by the microsporidia of Vairimorpha apis and V. ceranae. Obligate parasites of honey bees from the genus Apis colonize the insect’s gut, bringing about serious pathological changes in the infected honey bee and often inflicting considerable losses on the family; indeed, the entire apiary may perish (Botias et al. 2013; Goblirsch et al. 2013; Goblirsch 2018; Higes et al. 2009; Lee et al. 2008).
The preparation that we have been examining—an ethanol extract of the nest carton of the jet black ant L. fuliginosus—is of natural origin. This is an extremely important aspect in the treatment of honey bees, which play such a crucial part in the environment and also supply humans with food and medicinal products (Potts et al. 2016). As the administration of antibiotics for treating diseased honey bees is prohibited in many countries, attempts are being made to apply products of natural origin that are non-toxic to honey bees and do not build up in honey (Maistrello et al. 2008; Chaimanee et al. 2021; Özkırım and Küçüközmen 2021). The extract concentrations were chosen on our preliminary studies in which we checked different concentrations of this extract.
Ant nest carton is a conglomerate of many materials (plant matter, mineral particles, honeydew) together with bacterial and fungal micro-organisms (Maschwitz and Hölldobler 1970; Kaczmarczyk-Ziemba et al. 2020) (Figure 1C). The extracted material is thus a mixture of a very large number of compounds, including bioactive ones. In their study of a mixture of plant extracts used to combat nosemosis, Asli Özkırım and Küçüközmen (2021) found that the activities of the individual components against microsporidia were much weaker than that of their mixture, an indication that synergism is taking place. The nest carton extract that we have been studying may very well act in a similar way.
Analysis of selected biochemical parameters of the ethanol extract obtained from the ant nests used in this study showed only small quantities of proteins and total sugars to be present in comparison with data obtained using an aqueous extract (Wagner et al. 2021). The ethanol extract contained a very high level of phenolic substances, probably a direct consequence of the organic extraction method. Different content of certain groups of bioactive substances is the main distinction between results presented here and the results obtained using aqueous extract against nosemosis (Kunat et al. 2020). The presence of these substances was also confirmed by the FTIR analysis and correlated with the preparation’s high antioxidant activity. Phenolic components are potential free radical scavengers (Jaszek et al. 2013; Matuszewska et al. 2018). The FTIR analysis also indicated the presence of fatty acids, triglycerides, formic acid, lignin-derived phenolic compounds, and cellulose (Bhulaidok et al. 2010; Sihamala et al. 2010; Kristiansen and Amelung 2001; Liu and Kim 2017; Szymanska-Chargot and Zdunek 2013; Naz et al. 2016). The results are consistent with the fact that these compounds are not only ant body secretions but also components of the carton structure from which the preparation was obtained. Our data on the presence of lignin-derived phenols in the ethanol ant nest extracts concur with the results published by Kristiansen and Amelung (2001). The small band at 895 cm−1 indicates the probable presence of the β-glycosidic bond detected in cellulose chains (Liu and Kim 2017; Szymanska-Chargot and Zdunek 2013). This is due to the partial solubility of cellulose in ethanol (Naz et al. 2016), a small quantity of which may have been extracted from the nest material. The derivatives found in the preparation also exhibit antimicrobial activity: phenolic compounds, for example, may be involved in protective and de-activating mechanisms acting against Vairimorpha ceranae infections.
The expression of genes coding immune peptides in honey bees infected with Vairimorpha ceranae can either be attenuated (Antúnez et al. 2009) or stimulated (Chaimanee et al. 2012). Which result is obtained may depend on the methodology applied: for example, whether gene expression was examined on the basis of the entire organism of the honey bee, or solely a specific tagma, e.g., the abdomen, as was the case in the study by Chaimanee et al. (2012) and also in our experiment. It is well known that the particular types of some immune peptides like defensin are produced in different parts of the honey bee’s body. For instance, defensin-1 is secreted by glands in the head, whereas defensin-2 arises in the fat body in the abdomen (Ilyasov et al. 2012). Different methodologies can therefore yield quite disparate results.
Genes encoding immune peptides, i.e., abaecin, defensin-1, and hymenoptecin, which have antifungal (Vairimorpha spp.) and antibacterial (Paenibacillus larvae) properties, were selected for the experiments (Trytek et al. 2022). In our study, the levels of abaecin, defensin-1, and hymenoptaecin in the control group did not vary significantly during the experiment. In contrast, expression increased distinctly in most of the experimental variants, indicating that the extract positively stimulates the honey bee’s immune system, thereby significantly strengthening its immune response (Bíliková et al. 2001). This is a very considerable benefit of our product in comparison with antibiotics, which have an immunosuppressive effect on honey bees (Miyagi et al. 2000). A similar situation occurred in experiments performed by Byhrø et al. (2019), in which nurse and forager honey bees were given the bacterial cell wall components lipopolysaccharide (LPS) and peptidoglycan (PGN); thereafter, the expression levels of genes encoding defensin-1 and hymenoptaecin were examined. The expression of the defensin-1 gene was found to have increased in nurse honey bees after treatment with PGN but in forager honey bees after treatment with LPS. Moreover, the level of hymenoptaecin expression in forager honey bees increased after LPS and PGN treatment but did not change in nurse honey bees. Byhrø et al. (2019) demonstrated that nurse and forager honey bees regulate gene expression depending on the immunogen used. On the other hand, Glavinić et al. (2022) showed that supplementation with a natural substance, e.g., thymol, increased the immune level of infected honey bees and reduced the number of Vairimorpha spores, as was the case in our experiment. Among other things, however, this was observed in healthy honey bees given thymol, which decreases oxidative capacity and downregulates some immune-related gene expressions.
The search for a substance that would be beneficial for honey bees should be based mainly on natural substances. Our preparation, which is based on such substances, raises the level of immune gene expression in insects. It can therefore be regarded as an effective means of combatting nosemosis in honey bees.
Data availability
The article has no associated data.
Code availability
Not applicable.
References
Ahmed MK, McLeod MP, Nézivar J, Giuliani AW (2010) Fourier transform infrared and near-infrared spectroscopic methods for the detection of toxic diethylene glycol (DEG) contaminant in glycerin based cough syrup. Spectroscopy 24:601–608. https://doi.org/10.3233/SPE-2010-0482
Antúnez K, Martín-Hernández R, Prieto L, Meana A, Zunino P, Higes M (2009) Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ Microbiol 11:2284–2290
Bailey IW (1920) Some relations between ants and fungi ecology, 1(3):174–189. https://www.jstor.org/stable/1929134
Balachandran V, Parimala K (2012) Vanillin and isovanillin: comparative vibrational spectroscopic studies, conformational stability and NLO properties by density functional theory calculations. Spectrochim Acta A Mol Biomol Spectrosc 95:354–368. https://doi.org/10.1016/j.saa.2012.03.087
Bhulaidok S, Sihamala O, Shen L, Duo L (2010) Nutritional and fatty acid profiles of sun-dried edible black ants (Polyrhachis vicina Roger). Maejo Int J Sci Technol 4(2010):101–112
Bíliková K, Wu G, Šimúth J (2001) Isolation of a peptide fraction from honeybee royal jelly as a potential antifoulbrood factor. Apidologie, Springer Verlag 32(3):275–283. https://doi.org/10.1051/apido:2001129. hal-00891664
Boomsma JJ, Schimid-Hempel P, Hughes WOH (2005) Life histories and parasite pressure across the major groups of social insects. [In:] Insect Evolutionary Ecology, (eds) M. D. E. Fellowes, G. Holloway, and J. Rolff (Wallingford: CABI Publishing), 139–173
Botías C, Martín-Hernández R, Barrios L, Meana A, Higes M (2013) Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet Res 44(1):25. https://doi.org/10.1186/1297-9716-44-25
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Brinker P, Weig A, Rambold G, Feldhaar H, Tragust S (2019) Microbial community composition of nest carton and adjoining soil of the ant lasius fuliginosus and the role of host secretions in structuring microbial communities. Fungal Ecol 38:44–53. https://doi.org/10.1016/j.funeco.2018.08.007
Byhrø EMH, Salmela H, Vitlic A, Wang Y, Münch D, Amdam GV (2019) Different activation of immune-related genes in honey bee nurses and foragers (Apis mellifera). Apidologie 50:463–471. https://doi.org/10.1007/s13592-019-00658-z
Calderone NW (2012) Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992–2009. PLoS ONE 7(5):e37235. https://doi.org/10.1371/journal.pone.0037235
Chaimanee V, Chantawannakul P, Chen Y, Evans JD, Pettis JS (2012) Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae. J Insect Physiol 58(2012):1090–1095
Chaimanee V, Kasem A, Nuanjohn T, Boonmee T, Siangsuepchart A, Malaithong W, Sinpoo C, Disayathanoowat T, Pettis JS (2021) Natural extracts as potential control agents for Nosema ceranae infection in honeybees, Apis mellifera. J Invertebr Pathol 186. ISSN 107688:0022-2011. https://doi.org/10.1016/j.jip.2021.107688
Currie CR (2001) A community of ants, fungi, and bacteria: a multilateral approach to studying symbiosis. Annu Rev Microbiol 55:357–380. https://doi.org/10.1146/annurev.micro.55.1.357
Currie CR, Scott JA, Summerbell RC, Malloch D (1999) Fungus-growing ants use antibiotic-producing bacteria to control garden parasite. Nature 398:701–704. https://doi.org/10.1038/19519
Currie CR, Stuart AE (2001) Weeding and grooming of pathogens in agriculture by ants. Proc R Soc B 268:1033–1039. https://doi.org/10.1098/rspb.2001.1605
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Duo-Chuan L (2006) Review of fungal chitinases. Mycopathologia 161(6):345–360. https://doi.org/10.1007/s11046-006-0024-y. PMID: 16761182 42
Emsen B, Guzman-Novoa E, Hamiduzzaman MM, Eccles L, Lacey B, Ruiz-Pérez RA, Nasr M (2016) Higher prevalence and levels of Nosema ceranae than Nosema apis infections in Canadian honey bee colonies. Parasitol Res 115:175–181. https://doi.org/10.1007/s00436-015-4733-3
European Commission (2009) Commission Regulation No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No. 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No. 726/2004 of the European Parliament and of the Council. Official Journal of the European Union, L152/11L152/22
Glavinić U, Blagojević J, Ristanić M, Stevanović J, Lakić N, Mirilović M, Stanimirović Z (2022) Use of thymol in Nosema ceranae control and health improvement of infected honey bees. Insects 13(7):574. https://doi.org/10.3390/insects13070574
Goblirsch M (2018) Nosema ceranae disease of the honey bee (Apis mellifera). Apidologie 49:131–150. https://doi.org/10.1007/s13592-017-0535-1
Goblirsch M, Huang ZY, Spivak M (2013) Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 8:e58165. https://doi.org/10.1371/journal.pone.0058165
Goes AC, Barcoto MO, Kooij PW, Bueno OC, Rodrigues A (2020) How do leaf-cutting ants recognize antagonistic microbes in their fungal crops? Front Ecol Evol 8:95. https://doi.org/10.3389/fevo.2020.00095
Higes M, Martín-Hernández R, Garrido-Bailón E, González-Porto AV, García-Palencia P, Meana A, Del Nozal MJ, Mayo R, Bernal JL (2009) Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ Microbiol Rep 1:110–113. https://doi.org/10.1111/j.1758-2229.2009.00014.x
Ilyasov RA, Gaifullina LR, Saltykova ES, Poskryakov AV, Nikolenko AG (2012) Review of the expression of antimicrobial peptide defensin in honey bees Apis mellifera L. J Apic Sci 56(1):115–124. https://doi.org/10.2478/v10289-012-0013-y
Jaszek M, Osińska-Jaroszuk M, Janusz G, Matuszewska A, Stefaniuk D, Sulej J, Polak J, Ruminowicz M, Grzywnowicz K, Jarosz-Wilkołazka A (2013) New bioactive fungal molecules with high antioxidant and antimicrobial capacity Isolated from Cerrena Unicolor Idiophasic Cultures. Biomed Res Int 497492. https://dx.doi.org/10.1155/2013/497492
Jaszek M, Żuchowski J, Dajczak K, Cimek M, Grąz M, Grzywnowicz K (2006) Ligninolytic enzymes can participate in a multiple response system to oxidative stress in white-rot basidiomycetes: Fomes fomentarius and Tyromyces pubescens. Int Biodeterior Biodegradation 58:168–175. https://doi.org/10.1016/j.ibiod.2006.06.012
Kaczmarczyk-Ziemba A, Zagaja M, Wagner GK, Pietrykowska-Tudruj E, Staniec B (2020) The microbiota of the Lasius fuliginosus – Pella laticollis myrmecophilous interaction. Eur Zool J 87(1):754–769. https://doi.org/10.1080/24750263.2020.1844322
Krishanti NPRA, Zulfiana D, Wikantyoso B, Zulfitri A, Yusuf S (2018) Antimicrobial production by an Actinomycetes isolated from the termite nest. J Trop Life Sci 8(3):279–288. https://doi.org/10.11594/jtls.08.03.10
Kristiansen SM, W. Amelung W. (2001) Abandoned anthills of Formica polyctena and soil heterogeneity in a temperate deciduous forest: morphology and organic matter composition. Eur J Soil Sci 52:355–363. https://doi.org/10.1046/j.1365-2389.2001.00390.x
Kunat M, Wagner GK, Staniec B, Jaszek M, Matuszewska A, Stefaniuk D, Ptaszyńska AA (2020) Aqueous extracts of jet-black ant Lasius fuliginosus nests for controlling nosemosis, a disease of honeybees caused by fungi of the genus Nosema. Eur Zool J 87(1):770–780. https://doi.org/10.1080/24750263.2020.1845405
Lee SC, Corradi N, Byrnes EJIII, Torres-Martinez S, Dietrich FS, Keeling PJ, Heitman J (2008) Microsporidia evolved from ancestral sexual fungi. Curr Biol 18:1675–1679. https://doi.org/10.1016/j.cub.2008.09.030
Liu Y, Kim HJ (2017) Fourier transform infrared spectroscopy (FT-IR) and simple algorithm analysis for rapid and non-destructive assessment of developmental cotton fibers. Sensors 17(2017):1469. https://doi.org/10.3390/s17071469
Malarczyk E (1989) Transformation of phenolic acids by Nocardia. Acta Microbiol Pol 38:45–53
Martín-Hernández R, Meana A, Prieto L, Salvador AM, Garrido-Bailón E, Higes M (2007) Outcome of colonization of Apis mellifera by Nosema ceranae. Appl Environ Microbiol 73:6331–6338. https://doi.org/10.1128/AEM.00270-07
Maschwitz U, Hölldobler B (1970) Der Kartonnestbau bei Lasius fuliginosus Latr. (Hym. Formicidae). Z Vgl Physiol 66:176–189. https://doi.org/10.1007/BF00297777
Maistrello L, Lodesani M, Costa C et al (2008) Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 39:436–445. https://doi.org/10.1051/apido:2008022
Matuszewska A, Jaszek M, Stefaniuk D, Ciszewski T, Matuszewski Ł (2018) Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus cerrena unicolor. PLoS ONE 13:e0197044. https://doi.org/10.1371/journal.pone.0197044
Miyagi T, Peng Ch YS, Chuang RY, Mussen EC, Spivak MS, Doi RH (2000) Verification of oxytetracycline-resistant American foulbrood pathogen Paenibacillus larvae in the United States. J Invertebr Pathol 75:95–96
Mueller UG, Rehner SA, Schultz TR (1998) The evolution of agriculture in ants. Science 281:2034e2038. https://doi.org/10.1126/science.281.5385.2034
Mueller UG, Schultz TR, Currie CR, Adams RM, Malloch D (2001) The origin of the attine ant-fungus mutualism. Q Rev Biol 76:169–197. https://doi.org/10.1086/393867
Naz S, Ahmad N, Akhtar J, Ahmad N, Ali A, Zia M (2016) Management of citrus waste by switching in the production of nanocellulose. IET Nanotechnology 10(2016):395–399. https://doi.org/10.1049/iet-nbt.2015.0116
Özkırım A, Küçüközmen B (2021) Application of herbal essential oil extract mixture for honey bees (Apis mellifera L.) against Nosema ceranae and Nosema apis. J Apic Sci 65(1):163–175. https://doi.org/10.2478/JAS-2021-0010
Paduch R, Matysik G, Wojciak-Kosior M, Kandefer-Szerszen M, Nowak-Kryska S-K, M, Niedziela P. (2008) Lamium album extracts express free radical scavenging and cytotoxic activities. Pol J Environ Stud 17(4):569–580
Paudel YP, Mackereth R, Hanley R, Qin W (2015) Honey bees (Apis Mellifera L.) and pollination issues: current status, impacts and potential drivers of decline. J Agric Sci 7:93. https://doi.org/10.5539/jas.v7n6p93
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45. https://doi.org/10.1093/nar/29.9.e45
Potts SG, Imperatriz-Fonseca V, Ngo HT, Aizen MA, Biesmeijer JC, Breeze TD, Dicks LV, Garibaldi LA, Hill R, Settele J, Vanbergen AJ (2016) Safeguarding pollinators and their values to human well-being. Nature 540:220–229. https://doi.org/10.1038/nature20588
Ptaszyńska AA (2022) A short guide to the sixth mass extinction – is the Anthropocene an extended suicide? Revista de Educacion 395(1):27–41. https://revistaeducacion.org/EDU/journals/published/1628853580_j47Wb.pdf
Ptaszyńska AA, Borsuk G, Mułenko W, Demetraki-Paleolog J (2014) Differentiation of Nosema apis and Nosema ceranae spores under scanning electron microscopy (SEM). J Apic Res 53(5):537–544. https://doi.org/10.3896/IBRA.1.53.5.02
Ptaszyńska AA, Paleolog J, Borsuk G (2016) Nosema ceranae infection promotes proliferation of yeasts in honey bee intestines. PLoS ONE 11(10):e0164477. https://doi.org/10.1371/journal.pone.0164477
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1992) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26(9–10):1231–1237. https://doi.org/10.1016/s0891-5849(98)00315-3
Seifert B (2018) The ants of Central and North Europe. Boxberg: Lutra 407
Sen R, Ishak HD, Estrada D, Dowd SE, Hong E, Mueller UG (2009) Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc Natl Acad Sci USA 2009(106):17805–17810. https://doi.org/10.1073/pnas.0904827106
Sidhu CS, Joshi NK (2016) Establishing wildflower pollinator habitats in agricultural farmland to provide multiple ecosystem services. Front Plant Sci 7:363. https://doi.org/10.3389/fpls.2016.00363
Sihamala O, Bhulaidok S, Li-rong S, Duo L (2010) Lipids and fatty acid composition of dried edible red and black ants. Agric Sci China 9(2010):1072–1077. https://doi.org/10.1016/S1671-2927(09)60192-9
Singh K, Kumar A (2018) Kinetics of complex formation of Fe(III) with syringic acid: Experimental and theoretical study. Food Chem 265:96–100. https://doi.org/10.1016/j.foodchem.2018.05.071
Stanley DA, Msweli SM, Johnson SD (2020) Native honeybees as flower visitors and pollinators in wild plant communities in a biodiversity hotspot. Ecosphere 11:e02957. https://doi.org/10.1002/ecs2.2957
Szymanska-Chargot M, Zdunek A (2013) Use of FT-IR spectra and PCA to the bulk characterization of cell wall residues of fruits and vegetables along a fraction process. Food Biophys 8(2013):29–42. https://doi.org/10.1007/s11483-012-9279-7
Trytek M, Buczek K, Zdybicka-Barabas A, Wojda I, Borsuk G, Cytryńska M, Lipke A, Gryko D (2022) Effect of amide protoporphyrin derivatives on immune response in Apis mellifera. Sci Rep 12:14406. https://doi.org/10.1038/s41598-022-18534-9
Van den Berg R, Haenen GRMM, van den Berg H, Bast A (1999) Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem 66(4):511–517. 41. https://doi.org/10.1016/s0308-8146(99)00089-8
Wagner GK, Jaszek M, Staniec B, Prendecka M, Pigoń D, Belcarz A, Stefaniuk D, Matuszewska A, Pietrykowska-Tudruj E, Zagaja M (2021) Lasius fuliginosus nest carton as a source of new promising bioactive extracts with chemopreventive potential. Int J Mol Sci 22:4392. https://doi.org/10.3390/ijms22094392
Ye L, Zhao S, Li Y, Jiang S, Zhao Y, Li J, Yan K, Wang X, Xiang W, Liu C (2017) Streptomyces Lasiicapitis Sp. Nov., an actinomycete that produces kanchanamycin, isolated from the head of an ant (Lasius Fuliginosus L.). Int J Syst Evol Microbiol 67:1529–1534. https://doi.org/10.1099/ijsem.0.001756
Funding
The research was financed by an internal grant in the Institute of Biological Sciences of the Maria Curie-Skłodowska University as a part of the project: The effect of an alcoholic extract obtained from a carton-nest of Lasius fuliginosus ant in combating nosemosis.
Author information
Authors and Affiliations
Contributions
Material collection and preparation: GKW, BS, DS, AM and MJ. Analysis: MK-B, AM, MJ, DS, JK, AB and AAP. The first draft of the manuscript: GKW. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Manuscript Editor: Yves Le Conte
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wagner, G.K., Kunat-Budzyńska, M., Staniec, B. et al. Effectiveness of an ethanol extract of jet black ant nests for combatting nosemosis in apiary honey bees. Apidologie 54, 57 (2023). https://doi.org/10.1007/s13592-023-01037-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-01037-5