Abstract
Honey bee (Apis mellifera L.) thermoregulation plays an integral part in their behaviour and physiology and has been shown to be vulnerable to the effects of neonicotinoid insecticides. Baseline thermotolerance of 53.8 °C (defined as LT50; temperature at which 50% mortality is recorded) was determined for this subspecies. We evaluated the influence of sublethal dosages of three widely used neonicotinoid insecticides, clothianidin, imidacloprid and thiamethoxam, on individual Apis mellifera scutellata thermotolerance, each evaluated at a range of increasing ambient temperatures. For all three neonicotinoid treatments, A. m. scutellata thermotolerance was decreased by more than 3 °C as compared to the baseline data. Such a reduction in honey bee thermotolerance, especially under the increasing frequency and intensity of hot weather events, is a cause for concern when considering legislation and use of these neonicotinoids in the South African agricultural and suburban setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The honey bee, Apis mellifera L., is of great importance to both agricultural and natural ecosystems. Honey bees play a vital role in our food security (reviewed in Steffan-Dewenter et al. 2005), with the production of approximately one-third of all food crops relying on honey bee pollination (Morse and Calderone 2000). Of equal importance is the contribution of these pollinators to biodiversity and ecosystem function (Vanbergen and Insect Pollinators Initiative 2013). Worldwide declines in honey bee numbers and colony health are of particular concern, and while the exact causes of colony losses are still unclear, several factors appear to be influential, including climate change (Ruttner 1988; Le Conte and Navajas 2008), anthropogenic activities (Søvik et al. 2015), poor beekeeping practices (Gajger et al. 2010), habitat loss (Potts et al. 2010), monoculture (Kremen et al. 2002), introduction and prevalence of parasites (Bowen-Walker et al. 1999; Amdam et al. 2004), loss of genetic diversity (Meixner et al. 2010) and the use of pesticides (Holder et al. 2018), particularly neonicotinoid insecticides (Abbo et al. 2017; Calvo-Agudo et al. 2019).
Neonicotinoid insecticides are classified as systemic insecticides with neurotoxic properties, acting as an agonist on insect nicotinic acetylcholine (nAChR) receptors (Iwasa et al. 2004) which provide the majority of excitatory neurotransmissions in the insect central nervous system (Moffat et al. 2016). Neonicotinoids have a wide range of target pests and applications (Aliouane et al. 2009; Jeschke et al. 2011). The use of this class of insecticide has grown steadily worldwide since the 1990s, with various neonicotinoid insecticides registered for use in more than 120 countries, contributing considerably to global insecticide sales (ASSAf 2019). Neonicotinoid insecticides constitute around 25% of global insecticide sales, with the three most prominent active ingredients imidacloprid (hereafter IMI), clothianidin (hereafter CLO) and thiamethoxam (hereafter THX) specifically accounting for approximately 85% of total neonicotinoid sales for use in crop protection in 2012 (Bass et al. 2015).
The neurotoxic effects of neonicotinoids have been shown to influence a number of aspects of honey bee physiology and behaviour (Pettis et al. 2012) including hyperactivity (Suchail et al. 2001), communication of the waggle dance (Kirchner 1999; Schmuck 1999), flight muscle function, efficiency and coordination (Blanken et al. 2015), decreased social immune response such as grooming behaviour (Morfin et al. 2019), reduced immune-competence and impaired disease resistance (Brandt et al. 2016), food collection (Rortais et al. 2005), sucrose perception (Démares et al. 2016) and honey bee thermoregulation (Tosi et al. 2016).
Individual thermoregulation plays a crucial role in communication, social interaction and foraging activities (Stabentheiner and Hagmüller 1991; Stabentheiner et al. 1995, 2007) and involves the tetanic contraction of the flight muscles (thermogenesis) (Belzunces et al. 2012). Worker bees utilise their flight muscles during ‘shivering’ to generate heat and carry out brood warming and flight activities (Goller et al. 1991; Basile et al. 2008). They have also shown the ability to raise thorax temperatures to approximately 50 °C for brief periods (Stabentheiner et al. 2007). Dance communication in the hive also incorporates thermoregulation, for example food sources of higher sugar content are communicated by hotter body temperatures (Stabentheiner and Hagmüller 1991; Stabentheiner et al. 1995). Altered honey bee sucrose perception (Démares et al. 2016) and negatively impacted thorax temperature and heat generation (Tosi et al. 2016) through neonicotinoid exposure may detrimentally affect hive communication of food sources through the waggle dance (Tosi et al. 2016). Overall, honey bees have been found to be highly tolerant of heat stress, surviving well at temperatures ranging from 4 to 45 °C with some mortalities only at 50 °C (Koo et al. 2015). Evidence already exists of neonicotinoids affecting honey bees at higher temperatures, with the gene expression levels of the heat shock proteins hsp70, hsp78, and hsp90 shown to decrease with exposure to increasing concentrations of IMI (Koo et al. 2015). Individual thermoregulation is therefore reliant on optimal flight muscles function, evaporative cooling and the influence of external ambient temperatures (Bernd 1979). Under conditions of elevated or more frequently fluctuating ambient temperatures, the continued exposure to neonicotinoids, even at sublethal levels, neonicotinoid exposure can affect wing fanning and the ability of the bees to thermoregulate, thus, their ability to tolerate high temperatures.
The influence of external ambient temperatures on thermoregulation is one that is likely to vary between the various honey bee species and numerous subspecies, particularly within the western honey bee species which has a wide-ranging geographic distribution and a presence on several continents (Crane 2009). The natural distribution of A. mellifera L. extends through much of Africa, Europe and western Asia (Kotthoff et al. 2013). As a result, the subspecies existing in the temperate and Mediterranean type climates are likely to experience more stress within the lower extremes of their range of temperature tolerance, whereas those subspecies existing in warmer and drier climates, e.g. areas in their African distribution (Hepburn et al. 1998; Pirk 2020), are likely to experience more temperature tolerance stress towards the upper end of their tolerance range. Understanding the effects of rising temperature is even more crucial in Africa since the majority of the African populations are wild (Pirk et al. 2017) and therefore do not have a beekeeper who could put mitigation measures into place, like moving hives into shaded and cooler areas or away from potential pesticides exposures. For this reason, investigation into the limits of temperature tolerance of individual subspecies will better allow us to understand how future changes to large-scale climate conditions are likely to affect the honey bee species as a whole.
A previous study evaluating critical thermal limit and lethal temperature under conditions of increasing ambient temperature was conducted on two European honey bee subspecies: Apis mellifera ligustica M.M. Spinola, 1806, and Apis mellifera carnica Pollmann, 1879, in order to ascertain whether any differences between the thermal capacities of the two subspecies populations existed (Kovac et al. 2014). Our study further explored the lethal temperature aspect of honey bee physiology, by examining the previously undefined lethal thermal limits of African honey bees, Apis mellifera scutellata Lepeletier 1836. We established a threshold lethal thermal limit for A. m. scutellata as well as make a European subspecies comparison. Our study included the additional influence of three commercially prominent neonicotinoid insecticides on the ability of this honey bee subspecies to thermoregulate at elevated ambient temperatures in order to establish whether these insecticides influenced the lethal temperature threshold of this A. m. scutellata subspecies. The possible implications of the use of neonicotinoid insecticides on the African continent and the rapidly changing global climatic conditions on bee health were discussed.
2 Materials and methods
2.1 Study species
The interacting effects of neonicotinoids and ambient temperature on individual honey bee thermoregulation were conducted on A. m. scutellata. This subspecies is naturally distributed across sub-Saharan Africa (Ruttner 1988; Hepburn et al. 1998; Pirk 2020), and in South Africa specifically, the natural distribution covers the majority of the country with the exception of the Cape region which is home to the Apis mellifera capensis subspecies (Ruttner 1988; Crewe et al. 1994). Honey bees were collected from the Social Insect Research Group (SIRG) apiary located at the University of Pretoria’s Experimental Farm in Hatfield, Pretoria, Gauteng Province, South Africa, from May to August of 2016 and July to December of 2017. At the time of collection, experimental hives were free of obvious signs of disease and deemed sufficiently large to tolerate continuous removal of small numbers of honey bees. Hives were kept within city limits and not exposed to commercial agriculture agrochemicals.
The study focused on pollen and nectar/water foragers as they are easily detectable at hive entrances, experience the greatest variation in external temperature and the greatest potential exposure to environmental neonicotinoids while foraging. Not only do foragers consume pollen and nectar potentially contaminated with neonicotinoids, but they also become covered in it while foraging (Rortais et al. 2005). This puts them at risk of both topical and oral exposure (Rortais et al. 2005). Foragers are generally workers over the age of 21 days (Lindauer and Watkin 1953).
2.2 Baseline thermal tolerance
The baseline thermal tolerance of A. m. scutellata was established as per Kovac et al. (2014) with modifications. After collection, honey bee foragers were maintained in hoarding cages made of Perspex (120 mm × 95 mm × 80 mm) with sliding panels on both sides, a perforated panel for ventilation on the bottom and two small windows on the front to accommodate the insertion of two 4-mL Eppendorf tubes used to administer the diet (Köhler et al. 2013). No comb was provided during the temperature treatment in order to eliminate the influence of the comb’s microclimate on thermoregulation. For each trial, cages were kept for 24 h under controlled conditions, i.e. provided with two 2-mL Eppendorf tubes standard 50% w/w sugar water solution and maintained at standard hive conditions of 30 °C, 45% relative humidity (RH) (Kovac et al. 2014). Eppendorf tubes were weighed before going into the cage and again after the 24-h period, before the temperature ramp exposure. One cage per each of the 5 hives, consisting of 30 bees per cage, was evaluated at each of the 11 target temperatures, totalling 55 cages. For the first trial, the target temperature was 46 °C, 47 °C for the second, 48 °C for the third and so on at 1 °C intervals with the eleventh and final trial terminating at 56 °C. Each set of 5 cages was exposed to a temperature ramp, all starting an initial temperature of 30 °C and each trial ending at a different end temperature. At the start of each temperature trial, the cages were incubated at the starting 30 °C for 5 min and then the temperature was increased gradually by intervals of 0.3 °C min−1, terminating at the designated target temperature. Once the target temperature for the trial is reached, the cages were allowed to incubate at the target temperature for a brief stabilisation period of 5 min, cooled to the start temperature of 30 °C and incubated at this temperature for a further 8 h. The total mortality percentage for each cage was recorded directly after the temperature ramp treatment (considered 0 h), then again at 2 h, 4 h, 6 h and 8 h following temperature ramp respectively. All temperature experiments were conducted using temperature-controlled humidity chambers (HCP108 Memmert® GmbH + Co.KG), capable of carefully controlling temperature and humidity conditions. The rising temperatures during temperature ramps and the humidity conditions were controlled by the programme Celsius®, specifically designed Memmert® software with which interior chamber conditions could be pre-programmed. The mortality percentages from these 11 trials (150 honey bees per trial, 1650 honey bees in total) gave the baseline LT50 (LT50 considered the temperature at which a 50% mortality was recorded (Kovac et al. 2014)) of A. m. scutellata. Based on the baseline, we defined the range of relevant temperatures (deemed to be between 52 °C and 56 °C) at which to test the effects of the three individual neonicotinoids via oral exposure, at sublethal concentrations.
2.3 Neonicotinoid exposure
Three commercially utilised neonicotinoid insecticide active ingredients were used in the individual thermoregulation study, namely IMI, THX and CLO. Forager bees were maintained under the same conditions as for the baseline experiment above. Foragers used in the neonicotinoid trials were also provided with two 2-mL Eppendorf tubes of sugar solution per cage, both treated with the same sublethal dose of the relevant neonicotinoid (40 μL of 250 nM solution in 2 mL tube of 1:1 w/w sucrose and water, with the final concentration of given neonicotinoid being 5 nM), for a period of 24 h. Cages with bees exposed to neonicotinoids were exposed to the same temperature ramp methods as described in the baseline experiment. According to standard practice, acetone (hereafter ACE) was used as an organic solvent for the three neonicotinoids to make them soluble in the diet, with the proportion of ACE present in each diet, including the control, which was lower than 0.05% (Aliouane et al. 2009; Démares et al. 2016). The neonicotinoid concentration of 5 nM for CLO, IMI and THX respectively was considered comparable to realistic field doses; the period of exposure was sufficient to allow for all honey bees to consume sufficient treated sugar water to illicit any potential observable effects, though still remaining under the LD50 dosage of neonicotinoids (Démares et al. 2018; Yao et al. 2018).
Each individual neonicotinoid temperature ramp trial consisted of nine experimental cages. Two cages were each treated with IMI, CLO and THX respectively (40 μL of neonicotinoid in 2 × 2-mL tube of 1:1 w/w sucrose and water). Two controls were also included: one cage with the control sugar water solution only (SUC), and one control with sugar water and ACE (40 μL of a dilute ACE solution in 2 mL tube of 1:1 w/w sucrose and water). One cage with the control sucrose solution and no honey bees was included to measure and correct for the amount of evaporation over the 24-h exposure period. To ascertain the effects of neonicotinoids on this baseline LT50 threshold, 5040 honey bees from three hives treated with one of three separate neonicotinoids or two controls were then evaluated in the same way as the baseline. Mortality was assessed for all experiments at 0 h (Fig. 1), 2 h (Fig. 2), 4 h (Fig. 3), 6 h (Fig. 4) and 8 h (Fig. 5) following temperature ramp exposure.
Mean mortality of the honey bee A. m. scutellata recorded at 0 h following exposure to the designated temperature ramp which terminated at the indicated target temperature (°C), under control (ACE, red triangle, SUC, green diamond), baseline (Baseline, dark blue box) and treated (CLO, yellow circle; IMI, pink circle; THX, green circle) diet conditions. Functions could not be fitted for the 6 treatments. Error bars indicate standard deviation.
Mean mortality of the honey bee A. m. scutellata recorded at 2 h following exposure to the designated temperature ramp which terminated at the indicated target temperature (°C), under control (ACE, red triangle, SUC, green diamond), baseline (Baseline, dark blue box) and treated (CLO, yellow circle; IMI, pink circle; THX, green circle) diet conditions. Broken lines indicate lethal temperatures (LT50) determined from sigmoidal curves. Functions could not be fitted for the 6 treatments. Error bars indicate standard deviation.
Mean mortality of the honey bee A. m. scutellata recorded at 4 h following exposure to the designated temperature ramp which terminated at the indicated target temperature (°C), under control (ACE, red triangle, SUC, green diamond), baseline (Baseline, dark blue box) and treated (CLO, yellow circle; IMI, pink circle; THX, green circle) diet conditions. Broken lines indicate lethal temperatures (LT50) determined from sigmoidal curves. Curves were best fitted with a sigmoidal function (mortality = a/(1 + (x/b)c). Parameters for functions: Baseline (blue line), a = 7.097023E + 01, b = 5.428830E + 01, c = − 7.515814E + 01. CLO—function could not be fitted. IMI (pink line), a = 9.413888E + 01, b = 5.001369E + 01, c = − 9.733163E + 02. THX—function could not be fitted. ACE—function could not be fitted. SUC—function could not be fitted. Error bars indicate standard deviation.
Mean mortality of the honey bee A. m. scutellata recorded at 6 h following exposure to the designated temperature ramp which terminated at the indicated target temperature (°C), under control (ACE, red triangle, SUC, green diamond), baseline (Baseline, dark blue box) and treated (CLO, yellow circle; IMI, pink circle; THX, green circle) diet conditions. Broken lines indicate lethal temperatures (LT50) determined from sigmoidal curves. Curves were best fitted with a sigmoidal function (mortality = a/(1 + (x/b)c). Parameters for functions: Baseline (blue line), a = 9.228004E + 01, b = 5.408780E + 01, c = − 8.949900E + 01. CLO (yellow line), a = 1.304947E + 04, b = 1.469989E + 02, c = − 5.403435. IMI (pink line), a = 9.413888E + 01, b = 5.001369E + 01, c = − 9.733163E + 02. THX (green line), a = 6.422222E + 01, b = 4.998610E + 01, c = − 1.065781E + 03. ACE (red broken lines), a = 1.192658E + 02, b = 5.447768E + 01, c = − 1.1548067E + 01. SUC (light green line), a = 1.821269E + 04, b = 3.238560E + 02, c = − 3.190418E + 00. Error bars indicate standard deviation.
Mean mortality of the honey bee A. m. scutellata recorded at 8 h following exposure to the designated temperature ramp which terminated at the indicated target temperature (°C), under control (ACE, red triangle, SUC, green diamond), baseline (Baseline, dark blue box) and treated (CLO, yellow circle; IMI, pink circle; THX, green circle) diet conditions. Broken lines indicate lethal temperatures (LT50) determined from sigmoidal curves. Curves were best fitted with a sigmoidal function (mortality = a/(1 + (x/b)c). Parameters for functions: Baseline (blue line), a = 1.0344403E + 02, b = 5.3879726E + 01, c = − 7.70101459E + 01. CLO, a = 7.3722222E + 01, b = 4.998623E + 01, c = − 1.1532962E + 03. IMI (pink line), a = 8.333333E + 01, b = 4.9994453E + 01, c = − 1.08302836E + 03. THX, a = 7.958333E + 01, b = 4.9999488E + 01, c = − 1.0163530E + 03. ACE, a = 7.37777E + 01, b = 5.0050704E + 01, c = − 1.0680706E + 03. SUC, a = 7.2460823E + 01, b = 1.3080582E + 87, c = 4.0139539. Error bars indicate standard deviation.
The mortality percentages from these 7 trials (720 honey bees per trial, 5040 honey bees in total) were used to give an indication of the LT50 of A. m. scutellata, under the influence of three separate neonicotinoids via oral exposure, at sublethal concentrations.
For both the baseline and neonicotinoid experiments, a honey bee was considered to be alive when it moved, either spontaneously or in response to a gentle stimulus, and was assessed five times at 2-h intervals. Honey bees were provided with sugar syrup and water for the duration of the entire experiment, with the exception of the temperature ramp. This was because the evaporation from the diet, especially at higher temperatures, was found to significantly increase the humidity within the cages and thus the experimental conditions, making it impractical.
2.4 Statistics
Consumption data was evaluated for normality using a Shapiro–Wilk test (W = 0.97; p = 0.21). Mean consumption variation among the baseline, three treatments and two control groups were evaluated by an ANOVA and the data met all normality assumptions with a post hoc Tukey HSD test. A linear mixed effects model was used to determine whether consumption (response variable) varied among treatments including the baseline (predictor variable) to account for multiple measurements per hive; hive was included as the random effect. Models were fit using a maximum-likelihood approach and we used several plot types to assess model fit. The percentage of variance explained by the random effect, i.e. the hive number, was calculated by means of a variance component analysis (Crawley 2007). Models were fitted using the packages ‘nlme’ and ‘car’, in programme R (Fox and Weisberg 2011; Pinheiro et al. 2016; R Core Team 2021).
The recorded mortality percentage was plotted against the experimental target temperature and the lethal temperature (LT50) was ascertained by calculating the best-fitted sigmoidal curves. The effects of temperature on honey bee survival across all experiments were also evaluated using Kaplan–Meier survival analysis in Statistica © (version 13.2) and a Gehan’s Wilcoxon test used for pairwise comparison done to ascertain whether treatment affected honey bee survival at each of the various temperatures. An alpha value of 0.05 was used for all stats analysis (Pirk et al. 2013).
3 Results
A baseline thermal tolerance of was established for the A. m. scutellata subspecies (Baseline LT50, 8 h = 53.77 °C; N = 55 trials).
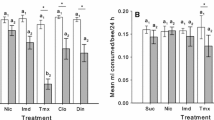
Following the 24-h exposure period, factoring in correction for evaporation, consumption of the treated diets (CLO, THX, IMI) and control diets (ACE, SUC) differed little from each other. Consumption across the different treatments was combined to determine the mean consumption per bee per treatment across all experiments following the standard 24 h of dietary exposure (Fig. 6). Mean consumption did vary but did not differ significantly (ANOVA df = 5; f value = 0.31; p value = 0.9) across the baseline, three treatments and two controls (Fig. 6).
The linear mixed effects model showed there were no differences in consumption between the different treatments (χ2 = 1.77; df = 5; p value = 0.88). Variation among hives explained only 1.85% of the variation in the model, meaning there is no variation among the hives, and we exclude hive as contributing factor for variance in the rest of the analyses.
Honey bee mortality showed a temperature dependence with increasing temperature, as well as a dependence with time across the 8-h period following the temperature ramp exposure. Mortality percentage data were best fitted with a sigmoidal function (mortality = a/(1 + (x/b)c) and an LT50 was determined from these fitted mortality curves. At the end of the 8-h period, the temperature treatment parameters were a = 1.0344403E + 02 and b = 5.3879726E + 01 and c = − 7.70101459E + 01 for baseline; a = 7.3722222E + 01 and b = 4.998623E + 01 and c = − 1.1532962E + 03 for CLO; a = 8.333333E + 01 and b = 4.9994453E + 01 and c = − 1.08302836E + 03 for IMI; a = 7.958333E + 01 and b = 4.9999488E + 01 and c = − 1.0163530E + 03 for THX; a = 7.37777E + 01 and b = 5.0050704E + 01 and c = − 1.0680706E + 03 for ACE; and a function could not be fitted for SUC (Fig. 5).
The lethal temperature after 8 h, derived from the sigmoidal curves, was not significantly different for the three neonicotinoid treatments (CLO LT50, 8 h = 50.15 °C; IMI LT50, 8 h = 50.05 °C; THX LT50, 8 h = 50.08 °C; N = 36 trials), slightly higher for both the ACE control (ACE LT50, 8 h = 50.50 °C; N = 18 trials) and the SUC control (SUC LT50, 8 h = 50.20 °C; N = 18 trials), and more than 3 °C higher for the Baseline control (baseline LT50, 8 h = 53.77 °C; N = 55 trials).
The mortality rates for the 5 treatments across the 8-h period following the temperature ramp exposure were compared for the lowest target temperature (50 °C) (Fig. S7) and the highest target temperature (56 °C) (Fig. S8). Mortality rate was higher and increased faster over time for all treatments at the higher temperature as opposed to the lower temperature.
Data was used to produce survival analysis graphs illustrating the survival rates of honey bees. These were recorded at 2-h intervals, over an 8-h period (0 h, 2 h, 4 h, 6 h, 8 h), per treatment (baseline, CLO, IMI, THX, SUC, ACE) across all seven target temperatures (50 to 56 °C) (Fig. S10–Fig. S16) as well as combined (Fig. S9). This comes after the bees’ exposure to individual temperature ramps. Overall, survival decreased more notable in treatments than the baseline (Fig. S9) whereas when separated per temperature, survival varied more at lower temperatures and decreased more uniformly at higher temperatures (Fig. S10–Fig. S16). Combined survival from all temperatures per treatment was compared for start and end observation time (0 h vs 8 h) to ascertain if time was a factor in survival. When comparing only trials from the neonicotinoid experiments, time was not found to be significant (Kaplan–Meier test χ2 = 7.628; df = 4; p = 0.106), but when survival data from both neonicotinoid and baseline experiment data were compared, time was found to be a significant factor influencing honey bee survival (Kaplan–Meier test χ2 = 326.528; df = 5; p < 0.000).
Survival data was then evaluated again over the same 8-h period for the combined five treatments, this time at each individual target temperature separately (Table I). Again, survival decreased over the 8-h period for all treatments and at all target temperatures, with all seven target temperatures having a significant effect on honey bee survival (Table I).
Survival for the baseline study was higher than the neonicotinoid treatments at the lower temperatures (50 to 53 °C) which lay above the LT50 values for the neonicotinoids. Survival for the baseline study at the higher temperatures (54 to 56 °C), which lies above the LT50 value for the baseline data, follows a similar trend to the survival of the neonicotinoid treatments. No significant difference was found between the survival of the baseline and the respective neonicotinoid treatments under these specific conditions.
Gehan’s Wilcoxon test was used for pairwise comparison to ascertain whether treatment affected honey bee survival at each of the various temperatures, with treatment differing significantly from baseline (p > 0.000) (Table SI).
4 Discussion
A baseline thermal tolerance of A. m. scutellata with an LT50 threshold (53.77 °C) was established for this subspecies. This LT50 was lowered by several degrees with exposure to sublethal doses of specific neonicotinoids. Mortality rate of these honey bees increased with increasing ambient temperature. While survival analysis also indicated both a decrease in survival over time and with increasing ambient temperatures, neonicotinoids did not appear to significantly affect survival rates.
Consumption of treated vs. control sucrose solutions over the 24-h exposure period did not differ significantly across the 5 treatments, suggesting no particular diet was preferred or avoided. Several studies have found little or no evidence that honey bees can taste or identify neonicotinoids in food sources (Kessler et al. 2015) although they do appear to affect the perception of sucrose (Démares et al. 2016).
Although survival decreased over time and with an increase in exposure temperature, neonicotinoids did not have a significant effect on mortality under these specific experimental conditions. A diet concentration of 50% w/w was used to ensure comparative consistency with the European study (Kovac et al. 2014); however, the high quality may play a role in the efficacy of the sublethal neonicotinoid dose. Honey bee forager survival has been found to show minimal impairment under good quality diet conditions (50% sugar solution) but exhibited far more harmful effects under conditions of increased nutritional stress (32.5% and 15% sugar solutions) (Tosi et al. 2017). For this study, the mortality data of 1650 individual honey bees across 5 experimental hives over 11 different temperature treatments were used to identify a baseline LT50 threshold for our A. m. scutellata study population, determined to be 53.77 °C. This is comparatively higher than the previously determined LT50 thresholds established for two European subspecies, A. m. carnica LT50 = 50.3 °C and A. m. ligustica LT50 = 51.7 °C (Kovac et al. 2014). Another study found similar differences between the Eastern (Asian) honey bee Apis cerana LT50 = 50.7 °C and the Western honey bee Apis mellifera LT50 = 51.8 °C in Yunnan, China, although no specific information on the A. mellifera population was provided in the study (Ken et al. 2005). For the two European subspecies, it was uncertain whether their physiology, their behaviour or a combination of both was responsible for their respective LT50 thresholds and the difference in thermal tolerance between the two subspecies, due mainly to the fact that they were provided with liquid food throughout the experiment enabling them to employ cooling behaviour (Kovac et al. 2014). The honey bees in our South African study were not provided with liquid food during the temperature ramp which may have limited their behavioural cooling ability, suggesting an even stronger resilience at higher temperatures compared to their European counterparts and might indicate a fundamental physiological base for such resilience. The differences in the adaptation of physiological limits to different climatic conditions is one possible explanation for the differences in these values between subspecies (Kovac et al. 2014).
Other comparative studies on lethal temperature between European subspecies yielded differing results to those of Kovac et al. (2014), which were attributed to differences in experimental methodologies. More specifically, the range of temperatures tested and the differing rates of temperature increase between the studies (Abou-Shaara et al. 2012; Kovac et al. 2014).
Results from our study are more comparable to the European study as the rate of increase, temperature range and relative humidity parameters were similar (Kovac et al. 2014). Difference identified in the LT50 values in both the European study and our own study among the three subspecies (A. m. carnica, A. m. ligustica and A. m. scutellata) may not necessarily be applicable to the respective subspecies as a whole, but rather the specific subspecies’ populations investigated (Kovac et al. 2014). This is owing to the morphological, behavioural and physiological adaptations to local conditions (Diniz-Filho et al. 2000; Alattal and AlGhamdi 2015).
Honey bees from both the European and South African studies were collected from a single region for each subspecies rather than several samples across the entire geographic distribution of each subspecies.
Samples from A. m. ligustica (Italian yellow bee) were collected from Emilia Romagna, Italy, which occurs in a warm, temperate climatic region with moderate temperatures (daytime annual average 12.9 °C) and significant rainfall throughout the year (Kovac et al. 2014). Samples from A. m. carnica (Carniolan honeybee) were collected from Styria, Austria, which is a cooler temperate region (daytime annual average 8.3 °C) and significant rainfall throughout the year (Kovac et al. 2014). Of the two European subspecies, A.m. ligustica occurs in the warmer of the two regions and recorded the higher of the two LT50 values suggesting a slightly higher threshold for heat tolerance.
The South African A. m. scutellata samples were all collected from the experimental apiary in the city of Pretoria in Gauteng, South Africa. No breeding activities take place at this site and it has been established from wild colonies therefore representing a wider geographical range (Moritz et al. 2007). Daytime annual average temperature for this region is 17.8 °C with mostly summer rainfall. This subspecies exhibited a higher LT50 value than either of the European subspecies, which could be attributed to the warmer, drier conditions and higher temperature extremes in their native region.
Neonicotinoid trials demonstrated a reduced LT50 for all three treatments in comparison to the baseline control LT50 data. CLO, IMI and THX LT50 values were more than 3 °C lower than the baseline LT50. The SUC and ACE controls from the neonicotinoid trials showed slightly more variation in their mortality and survival trend than the neonicotinoid trials but still echoed similar trends to the baseline control. This could be attributed to several factors, including differences in season. Predominantly, baseline trials were conducted in the autumn and early winter season, whereas neonicotinoid trials were conducted in the spring and summer seasons. The population demographics of Apis mellifera honey bees cycle in a seasonal manner in response to a colony’s needs, including adapting to the various challenges of winter (Bodenheimer 1937; Seeley and Visscher 1985). The longevity of spring bees (mean lifespan of 30–40 days) and summer bees (mean lifespan of 25–30 days) tends to be notably shorter than that of winter bees (mean lifespan in excess of 100 days, as long as 212–252 days) (Fukuda and Sekiguchi 1966; Mattila et al. 2001). This difference in seasonal longevity may be a contributing factor in the more fluctuating mortality of the spring/summer bees in the neonicotinoid control trials as compared to the autumn/winter bees used in the baseline trials.
The evidence that sub-lethal doses of three commercial neonicotinoids lower the already established LT50 thermal tolerance threshold for A. m. scutellata by several degrees adds to the growing evidence of the negative effects of these substances on important pollinators. Further fortification of this subspecies LT50 estimate should be done by conducting similar experiments on honey bees collected from more geographically diverse locations throughout its’ natural distribution range.
The lowering of the honey bee LT50 as a result of neonicotinoid exposure raises yet more concerns for the long-term survival of honey bees under the current and rapidly changing future climatic conditions on both a local and global scale. The evidence and effects of extreme heat events in several areas around the globe over the last few years continues to highlight the increasing environmental pressure that all species face. The African continent is considered particularly vulnerable to the effects of climate change and increasing heatwave frequency and intensity (Russo et al. 2016). The probability of heatwaves across the continent is predicted to continue to increase in the near future (Russo et al. 2016). Many areas of South Africa have experienced similar heatwaves to those recorded elsewhere, also with record-breaking intensity (Head 2018). Over the last 15 years, the probability of austral summer heatwaves in South Africa has notably increased as opposed to the period of 1961–1980 (Lyon 2009). Moving forward, the effects of neonicotinoids on aspects of honey bee thermoregulation should be further quantified, looking also to other areas in this subspecies geographical range as well as evaluating similar aspects of the neighbouring Cape bee subspecies, Apis mellifera capensis. This information is crucial when considering future legislation and the use of these neonicotinoids in the South African agricultural and suburban settings.
Data availability
Data will be made available on Research Gate.
Code availability
Not applicable.
References
Abbo PM, Kawasaki JK, Hamilton M, Cook SC, Degrandi-Hoffman G, Li WF, Liu J, Chen YP (2017) Effects of Imidacloprid and Varroa destructor on survival and health of European honey bees, Apis mellifera. Insect Sci 24:467–477. https://doi.org/10.1111/1744-7917.12335
Abou-Shaara HF, Al-Ghamdi AA, Mohamed AA (2012) Tolerance of two honey bee races to various temperature and relative humidity gradients. Environ Exp Biol 10:133–138
Academy of Science of South Africa (ASSAf) (2019) Neonicotinoids and their Impact on Ecosystem Services for Agriculture and Biodiversity in Africa. ASSAf Research Repository http://hdl.handle.net/20.500.11911/129. Accessed 18 Jan 2020
Alattal Y, AlGhamdi A (2015) Impact of temperature extremes on survival of indigenous and exotic honey bee subspecies, Apis mellifera, under desert and semiarid climates. Bull Insectology 68(2):219–222
Aliouane T, El Hassani AK, Gary V, Armengaud C, Lambin M, Gauthier M (2009) Subchronic exposure of honeybees to sublethal doses of pesticides: effects on behaviour. Environ Toxicol Chem 28:113–122. https://doi.org/10.1897/08-110.1
Amdam GV, Hartfelder K, Norberg K, Hagen A, Omholt SW (2004) Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during overwintering? J Econ Entomol 97:741–747. https://doi.org/10.1093/jee/97.3.741
Basile R, Pirk CWW, Tautz J (2008) Trophallactic activities in the honeybee brood nest – heaters get supplied with high performance fuel. Zool 111:433–441. https://doi.org/10.1016/j.zool.2007.11.002
Bass C, Denholm I, Williamson MS, Nauen R (2015) The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Phys 121:78–87. https://doi.org/10.1016/j.pestbp.2015.04.004
Belzunces LP, Tchamitchian S, Brunet JL (2012) Neural effects of insecticides in the honey bee. Apidologie 43(3):348–370. https://doi.org/10.1007/s13592-012-0134-0
Bernd H (1979) Thermoregulation of African and European honeybees during foraging, attack, and hive exits and returns. J Exp Biol 80:217–229. https://doi.org/10.1242/jeb.80.1.217
Blanken LJ, Van Langevelde F, Van Dooremalen C (2015) Interaction between Varroa destructor and imidacloprid reduces flight capacity of honeybees. Proc Royal Soc B 282(1820):20151738. https://doi.org/10.1098/rspb.2015.1738
Bodenheimer FS (1937) Studies in animal populations. II. Seasonal population-trends of the honey-bee. Q Rev Biol 12(4):406–425
Bowen-Walker PL, Martin SJ, Gunn A (1999) The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni. J Invertebr Pathol 73(1):101–106. https://doi.org/10.1006/jipa.1998.4807
Brandt A, Gorenflo A, Siede R, Meixner M, Büchler R (2016) The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J Insect Physiol 86:40–47. https://doi.org/10.1016/j.jinsphys.2016.01.001
Calvo-Agudo M, González-Cabrera J, Picó Y, Calatayud-Vernich P, Urbaneja A, Dicke M, Tena A (2019) Neonicotinoids in excretion product of phloem-feeding insects kill beneficial insects. Proc Natl Acad Sci USA 116(34):16878–16822. https://doi.org/10.1073/pnas.1904298116
Crane E (2009) Apis Species: (Honey Bees). In: Resh VH, Cardé RT (eds) Encyclopedia of insects. Academic Press, Cambridge, pp 31–32
Crawley MJ (2007) The R Book. Wiley, West Sussex
Crewe RM, Hepburn HR, Moritz RFA (1994) Morphometric analysis of 2 southern African races of honeybee. Apidologie 25(1):61–70. https://doi.org/10.1051/apido:19940107
Démares FJ, Pirk CW, Nicolson SW, Human H (2018) Neonicotinoids decrease sucrose responsiveness of honey bees at first contact. J Insect Physiol 108:25–30. https://doi.org/10.1016/j.jinsphys.2018.05.004
Démares FJ, Crous KL, Pirk CW, Nicolson SW, Human H (2016) Sucrose sensitivity of honey bees is differently affected by dietary protein and a neonicotinoid pesticide. PloS one: e0156584. https://doi.org/10.1371/journal.pone.0156584
Diniz-Filho JA, Hepburn HR, Radloff S, Fuchs S (2000) Spatial analysis of morphological variation in African honeybees (Apis mellifera L.) on a continental scale. Apidologie 31(2):191–204. https://doi.org/10.1051/apido:2000116
Fox J, Weisberg S (2011) An R companion to applied regression, Second. Sage, Thousand Oaks
Fukuda H, Sekiguchi K (1966) Seasonal change of the honeybee worker longevity in Sapporo, North Japan, with notes on some factors affecting the life-span. Jpn J Ecol 16(5):206–212. https://doi.org/10.18960/seitai.16.5_206
Gajger IT, Tomljanović Z, Petrinec Z (2010) Monitoring health status of Croatian honey bee colonies and possible reasons for winter losses. J Apicult Res 49(1):107–108
Goller F, Esch HE, Heinrich B (1991) How do bees shiver. Naturwissenschaften 78:325–328
Head T (2018) SA heatwave: 12 new temperature records have been set as mercury soars. The South African. https://www.thesouthafrican.com/news/sa-heatwave-temperature-records-september-2018. Accessed 29 July 2019
Hepburn HR, Radloff SE, Fuchs S (1998) Population structure and the interface between Apis mellifera capensis and Apis mellifera scutellata. Apidologie 29(4):333–346. https://doi.org/10.1051/apido:19980404
Holder PJ, Jones A, Tyler CR, Cresswell JE (2018) Fipronil pesticide as a suspect in historical mass mortalities of honey bees. P Natl Acad Sci USA 115(51):13033–13038. https://doi.org/10.1073/pnas.1804934115
Iwasa T, Motoyama N, Ambrose JT, Roe RM (2004) Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis Mellifera. Crop Prot 23(5):371–378. https://doi.org/10.1016/j.cropro.2003.08.018
Jeschke P, Nauen R, Schindler M, Elbert A (2011) Overview of the status and global strategy for neonicotinoids. J Agr Food Chem 59:2897–2908. https://doi.org/10.1021/jf101303g
Ken T, Hepburn HR, Radloff SE, Yusheng Y, Yiqiu L, Danyin Z, Neumann P (2005) Heat-balling wasps by honeybees. Naturwissenschaften 92(10):492–495. https://doi.org/10.1007/s00114-005-0026-5
Kessler SC, Tiedeken EJ, Simcock KL, Derveau S, Mitchell J, Softley S, Radcliffe A, Stout JC, Wright GA (2015) Bees prefer foods containing neonicotinoid pesticides. Nature 521(7550):74–76. https://doi.org/10.1038/nature14414
Kirchner WH (1999) Mad-bee-disease? Sublethal effects of imidacloprid (Gaucho) on the behaviour of honeybees. Apidologie 30:422
Köhler A, Nicolson SW, Pirk CW (2013) A new design for honey bee hoarding cages for laboratory experiments. J Apicult Res 52(2):12–14. https://doi.org/10.3896/IBRA.1.52.2.03
Koo J, Son TG, Kim SY, Lee KY (2015) Differential responses of Apis mellifera heat shock protein genes to heat shock, flower-thinning formulations, and imidacloprid. J Asia-Pac Entomol 18(3):583–589. https://doi.org/10.1016/j.aspen.2015.06.011
Kotthoff U, Wappler T, Engel MS (2013) Greater past disparity and diversity hints at ancient migrations of European honey bee lineages into Africa and Asia. J Biogeogr 40(10):1832–1838. https://doi.org/10.1111/jbi.12151
Kovac H, Käfer H, Stabentheiner A, Costa C (2014) Metabolism and upper thermal limits of Apis mellifera carnica and A.m. ligustica. Apidologie 45:664–677. https://doi.org/10.1007/s13592-014-0284-3
Kremen C, Williams NM, Thorp RW (2002) Crop pollinators from native bees at risk from agricultural intensification. P Natl Acad Sci USA 99(26):16812–16816. https://doi.org/10.1073/pnas.262413599
Le Conte Y, Navajas M (2008) Climate change: impact on honey bee populations and diseases. Rev Sci Tech OIE 27(2):499–510
Lindauer M, Watkin B (1953) Division of labour in the honeybee colony. Bee World 34(4):63–73. https://doi.org/10.1080/0005772X.1953.11094788
Lyon B (2009) Southern Africa summer drought and heat waves: observations and coupled model behavior. J Climate 22(22):6033–6046. https://doi.org/10.1175/2009JCLI3101.1
Mattila HR, Harris JL, Otis GW (2001) Timing of production of winter bees in honey bee (Apis mellifera) colonies. Insect Soc 48(2):88–93. https://doi.org/10.1007/PL00001764
Meixner MD, Costa C, Kryger P, Hatjina F, Bouga M, Ivanova E, Büchler R (2010) Conserving diversity and vitality for honey bee breeding. J Apicult Res 49(1):85–92. https://doi.org/10.3896/IBRA.1.49.1.12
Moffat C, Buckland ST, Samson AJ, McArthur R, Pino VC, Bollan KA, Huang JTJ, Connolly CN (2016) Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Sci Rep 6(1):1–10. https://doi.org/10.1038/srep24764
Morfin N, Goodwin PH, Hunt GJ, Guzman-Novoa E (2019) Effects of sublethal doses of clothianidin and/or V. destructor on honey bee (Apis mellifera) self-grooming behavior and associated gene expression. Sci Rep 9:5196. https://doi.org/10.1038/s41598-019-41365-0
Moritz RF, Kraus FB, Kryger P, Crewe RM (2007) The size of wild honeybee populations (Apis mellifera) and its implications for the conservation of honeybees. J Insect Conserv 11(4):391–397. https://doi.org/10.1007/s10841-006-9054-5
Morse RA, Calderone NW (2000) The value of honey bee pollination in the United States. Bee Cult 128:1–15
Pettis JS, Johnson J, Dively G (2012) Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 99(2):153–158. https://doi.org/10.1007/s00114-011-0881-1
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2016) Nlme: linear and nonlinear mixed effects models, R package version 3.1–128. http://cran.r-project.org/package=nlme
Pirk CWW (2020) African Honey Bees. In: Starr C (ed) Encyclopedia of Social Insects. Springer International Publishing, New York, pp 1–4
Pirk CWW, De Miranda JR, Kramer M, Murray TE, Nazzi F, Shutler D, Van der Steen JJ, Van Dooremalen C (2013) Statistical guidelines for Apis mellifera research. J Apic Res 52(4):1–24. https://doi.org/10.3896/IBRA.1.52.4.13
Pirk CWW, Crewe RM, Moritz RFA (2017) Risks and benefits of the biological interface between managed and wild bee pollinators. Funct Ecol 31(1):47–55. https://doi.org/10.1111/1365-2435.12768
Potts SG, Roberts SPM, Dean R, Marris G, Brown MA, Jones HR, Neumann P, Settele J (2010) Declines of managed honey bees and beekeepers in Europe. J Apic Res 49(1):15–22. https://doi.org/10.1007/s00114-011-0881-1
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 2021
Rortais A, Arnold G, Halm MP, Touffet-Briens F (2005) Modes of honeybees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie 36(1):71–83. https://doi.org/10.1051/apido:2004071
Russo S, Marchese AF, Sillmann J, Immé G (2016) When will unusual heat waves become normal in a warming Africa? Environ Res Lett 11(5):054016
Ruttner F (1988) Biogeography and taxonomy of honeybees. Springer Verlag, New York
Schmuck R (1999) No causal relationship between Gaucho® seed dressing in sunflowers and the French bee syndrome. Pflanzenschutz Nachrichten-Bayer-English Edition 52:257–299
Seeley TD, Visscher PK (1985) Survival of honeybees in cold climates: the critical timing of colony growth and reproduction. Ecol Entomol 10(1):81–88. https://doi.org/10.1111/j.1365-2311.1985.tb00537.x
Søvik E, Perry CJ, Lamora A, Barron AB, Ben-Shahar Y (2015) Negative impact of manganese on honeybee foraging. Biol Letters 11(3):20140989. https://doi.org/10.1098/rsbl.2014.0989
Stabentheiner A, Hagmüller K (1991) Sweet food means “hot dancing” in honeybees. Naturwissenschaften 78:471–473. https://doi.org/10.1007/BF01134389
Stabentheiner A, Kovac H, Hagmüller K (1995) Thermal behavior of round and wagtail dancing honeybees. J Comp Physiol B 165:433–444. https://doi.org/10.1007/BF00261297
Stabentheiner A, Kovac H, Schmaranzer S (2007) Thermal behaviour of honeybees during aggressive interactions. Ethology 113:995–1006. https://doi.org/10.1111/j.1439-0310.2007.01403.x
Steffan-Dewenter I, Potts SG, Packer L (2005) Pollinator diversity and crop pollination services are at risk. Trends Ecol Evol 20:651–652
Suchail S, Guez D, Belzunces LP (2001) Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ Toxicol Chem 20(11):2482–2486. https://doi.org/10.1002/etc.5620201113
Tosi S, Démares FJ, Nicolson SW, Medrzycki P, Pirk CW, Human H (2016) Effects of a neonicotinoid pesticide on thermoregulation of African honey bees (Apis mellifera scutellata). J Insect Physiol 93:56–63. https://doi.org/10.1016/j.jinsphys.2016.08.010
Tosi S, Nieh JC, Sgolastra F, Cabbri R, Medrzycki P (2017) Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. P Roy Soc B- Bio Sci 284(1869):20171711. https://doi.org/10.1098/rspb.2017.1711
Vanbergen AJ, Initiative IP (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ 11:251–259. https://doi.org/10.1890/120126
Yao J, Zhu YC, Adamczyk J (2018) Responses of honey bees to lethal and sublethal doses of formulated clothianidin alone and mixtures. J Econ Entomol 111:1517–1525. https://doi.org/10.1093/jee/toy140
Acknowledgements
The research was supported by the National Research Foundation (NRF). We would like to thank members of the Social Insects Research Group for assistance with sample collection.
Funding
Funding was provided by the South African National Research Foundation (NRF) and the University of Pretoria.
Author information
Authors and Affiliations
Contributions
Experimental design—LCB and CWWP. Data collection—LCB. Data analysis—LCB, MW and CWWP. Manuscript preparation—LCB, MW and CWWP. All authors contributed to the study concept and design. Material preparation and data collection were performed by Laura Catherine Bester; analysis was performed by Laura Catherine Bester, Mia Wege and Christian Walter Werner Pirk. Manuscript preparation and final approval were performed by Laura Catherine Bester, Mia Wege and Christian Walter Werner Pirk.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: James Nieh
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bester, L.C., Wege, M. & Pirk, C.W.W. The influence of sublethal neonicotinoid doses on individual Apis mellifera scutellata thermotolerance. Apidologie 54, 7 (2023). https://doi.org/10.1007/s13592-023-00989-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-00989-y