Abstract
Orchid bees (Euglossini) are pollinators sensitive to landscape pressures related to agricultural land use, such as coffee farming. Coffee crops occupy a large land area in Brazil, and understanding the effects of coffee farming on bee communities is essential to pollinator conservation in modified landscapes. Here, we evaluated the Euglossini communities in forest patches surrounded by coffee crops in the Atlantic Forest. We hypothesized the negative effects of coffee cover (%) on euglossine richness and abundance. The euglossine males were sampled at a sampling point within forest patches of 15 landscapes in southeastern Brazil. A total of 1890 euglossine males in four genera and 14 species were sampled. Eulaema nigrita Lepeletier, 1841 was the dominant species (55.1%), followed by Euglossa cordata (Linnaeus, 1758) (25.5%). We found a new record for Euglossa liopoda Dressler, 1982, increasing the species’ known range in the Atlantic Forest. The results showed that the euglossine richness and species abundance decreased in forest patches surrounded by a high coffee cover (%). These negative effects of coffee cover on the Euglossini communities are related to forest cover substitution by monocultures with low or no floral attractiveness for these bees. This study highlights that forest patches in agricultural landscapes sustain high levels of euglossine richness. Thus, we indicate the conservation importance of these Atlantic Forest patches for bee species requirements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Agricultural expansion and intensification are one of the main causes of habitat loss and fragmentation worldwide. These human activities affect the landscape structure through changes in patch types and spatial arrangement of these elements in the landscape (i.e., landscape composition and configuration, respectively) (Fischer and Lindenmayer 2007; Fahrig 2017). Moreover, the appearance of anthropogenic matrices (i.e., areas of anthropic use) can increase isolation between habitat patches (Driscoll et al. 2013), influencing species maintenance and essential ecological services in modified landscapes, such as pollination by bees (Kremen et al. 2007; Cariveau and Winfree 2015; Martello et al. 2016).
The orchid bees (Hymenoptera: Apidae: Euglossini) are a Neotropical group with more than 240 species (Roubik and Hanson 2004; Moure et al. 2012). These bees are fundamental to the ecosystem’s functioning through pollination, as well as are essential for pollination ecosystem services within agroecosystems (Roubik and Hanson 2004; Briggs et al. 2013). Different morphological and behavioral traits of Euglossini bees (e.g., long tongue, robust body, buzz behavior) make them important pollinators for many plant families (Cortopassi-Laurino et al. 2009; Rocha-Filho et al. 2012). Moreover, these bees have a broad flight range (Wikelski et al. 2010) and are pollen vectors of the plant species spatially distributed in the landscape (Janzen 1971).
For some reasons, Euglossini bees constitute important ecological indicators of environmental and landscape disturbances, including their forest dependence, wide geographic range, and easy sampling (Allen et al. 2019; Gonçalves and Faria 2021). These bees are quite sensitive to biotic and abiotic variable changes, such as phytophisiognomy type, altitude, temperature, and humidity (Aguiar and Gaglianone 2012; Sobreiro et al. 2019). Moreover, ecological attributes such as euglossine abundance show a high association with landscape structure (Allen et al. 2019; Carneiro et al. 2022), indicating that these bees are good ecological models for understanding bee responses to land-use changes (Brosi 2009; Allen et al. 2019; Gonçalves and Faria 2021). Several studies highlighted the influence of the landscape structure on Euglossini communities (e.g., Cândido et al. 2018; Allen et al. 2019; Opedal et al. 2020). These studies have indicated the influence of forest patch attributes and landscape context on euglossine richness and abundance (Brosi 2009; Carneiro et al. 2021; Sousa et al. 2022). For other bee groups and invertebrates — such as dung beetles, ants, wasps, and flies — anthropogenic matrices play an essential role in maintaining the richness and species abundance (Martello et al. 2016, 2022; Medeiros et al. 2021).
Landscape composition is important for euglossine bees because it can provide complementary habitats or niche opportunities for some of these bee species (Hedström et al. 2006a; Aguiar et al. 2015; Carneiro et al. 2021). There is a remarkable decrease in euglossine richness and abundance in matrices such as forestry (e.g., commercial Eucalyptus plantations) and coffee crops compared to forest areas (Briggs et al. 2013; Aguiar et al. 2015). Furthermore, highly managed agroecosystems are less friendly to euglossine species exhibiting a higher dependence on forested habitats (Milet-Pinheiro and Schlindwein 2005; Aguiar et al. 2015), and among many factors, this probably results from higher input of agrochemicals, such as fertilizers, insecticides, fungicides, and herbicides.
Although these orchid bees show consistent responses to agricultural landscape attributes (Allen et al. 2019; Carneiro et al. 2021), the influence of coffee cover (%) on euglossine communities is poorly understood (Briggs et al. 2013). Some studies in Central America indicated that coffee farming systems (e.g., monoculture, polyculture) affect the Euglossini community’s composition (Hedström et al. 2006a, b; Briggs et al. 2013). Brazil has been globally responsible for 34.9% (sd. 1.8%) of coffee production in the last 5 years (International Coffee Organization 2021). The cultivated area exceeds 2.2 million hectares, 1.9 million of them in southeastern Brazil (Conab 2021). Since Atlantic Forest originally covered this region, the expansion of coffee farming contributes to the high forest loss of this biodiversity hotspot. Thus, evaluating the influence of coffee cover (%) on euglossine communities can indicate the effects of forest cover loss related to agricultural expansion and intensification in tropical regions (Briggs et al. 2013; Dicks et al. 2021), and show paths to sustainable agriculture practices in modified landscapes combined with pollinator conservation.
Here, we evaluated the Euglossini community structure in 15 agricultural landscapes. We then analyzed how coffee cover (%) surrounding forest patches affects the euglossine richness and species abundance. Coffee species bloom a few times a year, and although many bee species benefit from floral resources during this short flowering period (Hipótilo et al. 2018), the use of these resources by Euglossini species remains unknown. For this reason, and considering that coffee farming can negatively affect the Euglossini communities (Hedström et al. 2006a; Briggs et al. 2013), we expected a decrease in bee richness and abundance in landscapes with high coffee cover (%).
2 Material and methods
2.1 Study area
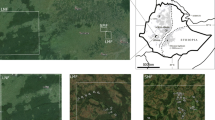
We surveyed 15 agricultural landscapes in southeastern Brazil (Fig. 1), located in the municipalities of Varre-Sai (Rio de Janeiro state; L01–L06), Espera Feliz (Minas Gerais state; L07–L09), Guaçuí, and Alegre (Espírito Santo state; L10–L15). This region has altitudes varying between 500 and 2000 m. There are crops of Coffea canephora Pierre ex A. Froehner in the lower altitude regions and Coffea arabica Linnaeus in the higher altitude areas. We delimited the landscapes along a gradient of forest cover (Carneiro et al. 2021) and coffee cover (0.07–55.85%) (Fig. 1). Detailed information about the study area can be found in Carneiro et al. (2021).
2.2 Euglossini bee sampling
We selected a forest patch in each agricultural landscape, with a minimum size of 5.0 ha, and surrounded by coffee crops (Fig. 1). We sampled the euglossine males within the forest patch at least 200 m distant from the forest edge. The sampling was carried out during three days in the rainy season (November 2019 to March 2020) and two days in the dry season (August to September 2019, July 2020) (Carneiro et al. 2021). We used traps with five baits to collect the euglossine males: eucalyptol, eugenol, methyl cinnamate, methyl salicylate, and vanillin. The sampling method is detailed in Carneiro et al. (2021) and Carneiro et al. (2022). The specimens have been deposited in the entomological collection of the Experimental Ecology sector, Laboratório de Ciências Ambientais—LCA, Universidade Estadual do Norte Fluminense Darcy Ribeiro—UENF.
2.3 Coffee cover quantification
We delimited the landscapes with 1500-m buffers from the bee sampling point, followed by vectorization and visual classification with satellite images in ArcGis software. From the rasterized map (5-m resolution) (Carneiro et al. 2021), we used the lsm function of the R landscapemetrics package (Hesselbarth et al. 2019) to quantify the coffee cover (%) in the landscape. For this, we used the PLAND landscape metric, which quantifies the class percentage in the landscape (McGarigal 2015). Because euglossine bees respond to landscape attributes in different spatial scales (Brosi 2009; Carneiro et al. 2022; Sousa et al. 2022), we used a multiscale approach to measure the coffee cover (%) in different spatial scales, from 300 to 1500 m, with 50-m intervals. In this range are included the scales within which landscape attributes have been shown to influence euglossine richness and abundance (i.e., the scale of effect) (Brosi 2009; Cândido et al. 2018; Carneiro et al. 2021).
2.4 Data analysis
We evaluated the Euglossine community structure through the Shannon–Wiener diversity (H′ = − ∑ pi lnpi), Berger-Parker dominance (D = Nmax/N), and Pielou uniformity (J′ = H′/ln(S)) indexes (Magurran 2004). We used rarefaction curves to correlate the number of sampled individuals with the species richness in each landscape. We plotted the rarefaction curves for each landscape group considering its geographic location (L01–L06; L07–L09; L10–L15, see Fig. 1) because there is a high variation in bee abundance between landscapes and to visualize the rarefaction curves better. These analyzes were performed with the Past 4.01 software (Hammer et al. 2001). The community composition similarity between the landscapes was quantified through Renkonen’s index, recommended for small samples (Wolda 1981). We used Renkonen’s values for an Unweighted Pair Group Method with Arithmetic Mean—UPGMA analysis. The UPGMA dendrogram was plotted from the small distances of Renkonen’s similarity. We used a bootstrap with 1000 randomizations to quantify the stability and reliability of the clusters in the UPGMA dendrogram. Both Renkonen index and UPGMA were calculated with the R R4eco package (available at: http://github.com/wilsonfrantine/R4eco).
We classified the species into common and rare using an inflection point and Gaston’s quartiles criteria (Gaston 1994; Siqueira et al. 2012). In these approaches, a relative abundance curve is used. From the curve shape change (i.e., inflection point), we visually defined the common species on the left side and rare species on the right side (Siqueira et al. 2012). Gaston’s quartile criterion classifies the 25% most abundant species as common and the 25% less abundant species as rare. However, we also considered the species distributed in second and third quartiles to avoid singletons and doubletons being included in the rare species group (Siqueira et al. 2012). Then, we classified the common species as the 25% most abundant species and the 75% less abundant as rare species (Fig. S1).
We performed a multiscale analysis similar to Gestich et al. (2018) and Ahuatzin et al. (2021) to previously determine the scale of effect of the coffee cover (%) on the euglossine richness, abundance of common and rare species. The scale of effect is the spatial scale of a landscape attribute that best explains biological parameters (Miguet et al. 2016). For this, we used the multifit R function (Huais 2018) with the R2 selection criteria in a nested multiscale approach (Carneiro et al. 2022). The scale of effect was presented with the highest R2 (Fig. S2, S3, S4). Afterward, we used General Linear Models with 5% significance to associate the euglossine community attributes with coffee cover (%) in the respective scales of effect. Before that, we log10 transformed the abundance variables to fit the normality assumptions. We used the boxcox function of the R MASS package for the model validation (Venables and Ripley 2002). These analyses were performed in the R 4.0.2 software (R Core Team 2020).
3 Results
3.1 Euglossini community structure
We sampled 1890 euglossine males belonging to four genera and 14 species (Table I). The relative abundance in each landscape ranged from 0.3 to 20.6%. The bee richness ranged from four to nine species (Table I). Eulaema nigrita Lepeletier, 1841 was sampled in all the 15 landscapes, with the highest relative abundance (> 50%) in 13 landscapes (excepted L08 = 14.2%; and L14 = 21.4%). Euglossa cordata (Linnaeus, 1758) was also sampled in all 15 landscapes and was the most abundant species (68.8%) in one of them. Eulaema atleticana Nemésio, 2009, Eufriesea gr. auriceps sp2, Euglossa cf. leucotricha Rebêlo and Moure, 1995, and Euglossa liopoda Dressler, 1982 were singletons in four landscapes (Table I). Among the 14 species, three were classified as common and 11 species as rare (Table I; Fig. S1). The highest richness of rare species (S = 6) occurred in two landscapes, while one of the landscapes presented just one rare species (S = 1) (Table I). The highest species diversity was H′ = 1.47, and the lowest was H′ = 0.8 (Table I). The highest equitability was J′ = 0.91, and the lowest was J′ = 0.44, while the highest species dominance was D = 0.79, and the lowest was D = 0.42 (Table I).
The rarefaction curves reached asymptote in four of 15 landscapes (Fig. 2a, c). We observed a curve slope change in the other rarefaction curves, although they have not stabilized, except for three curves, which were ascending (Fig. 2b). We found a higher similarity distance from Renkonen’s index in the L08 community composition regarding the other landscapes (Fig. 3). The lower distance of the community similarity was between L06 and L013, with the higher reliability and repetition with the bootstrap method (Fig. 3).
3.2 Influence of coffee cover (%) on Euglossini bees
The scale of effect of coffee cover (%) on the euglossine communities was at smaller spatial scales (between 300 and 400 m) (Fig. S2, S3, S4). We found a negative and statistically significant effect of coffee cover on species richness (R2 = 0.34; p = 0.020), abundance of common (R2 = 0.70; p < 0.001) and rare species (R2 = 0.60; p = 0.000) (Fig. 4a–c).
4 Discussion
We described the Euglossini communities in 15 landscapes associated with coffee farming in southeastern Brazil. The observed species richness (S = 14) was similar to that recorded in nearby areas of the semideciduous Atlantic Forest, such as southern Minas Gerais state (Silveira 2014; S = 14) and northern Rio de Janeiro state (Aguiar et al. 2014; S = 11 species). Euglossa liopoda has its first record below 20° S latitude, expanding the species’ geographical distribution about 250 km south of its southernmost records in the Atlantic Forest (Neves and Viana 1997; Nemésio 2009; Medeiros et al. 2017). Eulaema atleticana was recorded in one out of 15 landscapes, and this species has been observed as a singleton in the region (Aguiar and Gaglianone 2012). The northern portion of Rio de Janeiro state may be the current southern limit of this bee in the Atlantic Forest (Aguiar and Gaglianone 2012).
Eulaema nigrita and Euglossa cordata showed the highest relative abundances among the sampled species. These species occur in different ecosystems of the Atlantic Forest (Sofia and Suzuki 2004; Aguiar and Gaglianone 2012; Medeiros et al. 2017), Amazon Forest (Moure et al. 2012; Cândido et al. 2018), Brazilian Cerrado, and Seasonally Dry Tropical Forest (Martins et al. 2018; Carneiro et al. 2018; Sousa et al. 2022). Given this environmental plasticity, these two euglossine species were found at high abundance in open and perturbed landscapes (Tonhasca et al. 2002; Aguiar and Gaglianone 2008; Silva and De Marco 2014; Aguiar et al. 2015). On the other hand, Euglossa clausi Nemésio and Engel (2012), sampled in seven landscapes, is an endemic species of the Atlantic Forest (Nemésio and Engel 2012). This bee has been pointed out as a potential bioindicator of conserved ecosystems (Ramalho et al. 2009; Aguiar et al. 2015). Then, some agricultural landscapes with higher forest cover may be more friendly to euglossine populations with a forest dependence (Carneiro et al. 2022).
Community similarities were stable in most landscapes, except for two of them (L08 and L14). This result may be related to the landscapes’ shared dominance of E. nigrita, but these two landscapes showed a dominance change from E. nigrita to Euglossa truncata and E. cordata, respectively. While the low total abundance (L08, N = 7) was associated with the high altitudes (> 1200 m), the proximity of a urbanized matrix (L14) seems to be an important factor. E. cordata has been sampled in urban areas, exhibiting an exceptional adaptation in such environments through time (Rocha-Filho et al. 2020). On the other hand, similar sites present the landscape attributes shaping the community composition. The higher community similarity was between landscapes spatially distant (L06 and L13, ~ 30 km), but they share a low forest cover (11.5% and 12.5%, respectively) which may have influenced this result, as observed for other landscape components elsewhere (Cândido et al. 2018). These two landscapes also shared the same species composition and asymptotic rarefaction curves with few individuals (N < 50), indicating a low euglossine richness in both landscapes. In some scenarios, human-modified landscapes may favor euglossine species with higher environmental tolerance and wide spatial distribution (e.g., E. cordata, E. nigrita) (Cândido et al. 2018; Carneiro et al. 2021). Therefore, the landscape composition seems to shape the similarity among our sampling sites, with factors such as human land modifications driving these community patterns. In addition, within agroecosystem-dominated landscapes of Brazilian Cerrado, natural forest cover and fragmentation per se were the best predictors for Euglossini species richness (Sousa et al. 2022). However, in that study, the authors did not explore the effect of the two most common anthropogenic matrices (agriculture and pasture).
5 Concluding remarks
We confirmed the expectation that increased coffee cover (%) negatively affects euglossine richness and abundance. The coffee plants provide floral resources (i.e., nectar and pollen) for many bee species (Hipólito et al. 2018). However, these floral resources are unattractive for euglossine species (Briggs et al. 2013; Hipólito et al. 2018; Hautequestt unpubl. data). These orchid bees can explore floral resources in pioneer plants at coffee crops (Hautequestt unpubl. data), but these plants are withdrawn through high-intensity management. Moreover, some coffee plantations receive loads of agrochemicals throughout the year, such as fertilizer and herbicides. These factors may act synergistically, resulting in the negative effects of coffee cover on Euglossini bees.
The coffee cover in the spatial scales evaluated was negatively correlated with forest cover (Fig. S5). Then, the negative effects of coffee cover on Euglossini communities are linked to forest cover loss, especially in the focal forest patches. This indirectly corroborates other observations showing lower euglossine richness and abundance in smaller forest patches (Brosi 2009; Nemésio and Silveira 2010). Forest cover (%) also played an important role in maintaining species richness and abundance within modified landscapes of Brazilian Cerrado and Atlantic Forest (Sousa et al. 2022; Carneiro et al. 2022). In addition, fragmentation per se had a negative effect on these bees (Sousa et al. 2022). It is important to emphasize that the landscape heterogeneity on larger spatial scales in these agricultural areas positively influences the euglossine richness and abundance (Carneiro et al. 2021, 2022). On these spatial extensions, the negative effects of coffee cover may be diluted by a compositional heterogeneity associated to patch types functionally important for euglossine species (Aguiar et al. 2015; Carneiro et al. 2021). Our results show that — if extensive coffee areas replace this landscape heterogeneity — the local negative effects of the coffee cover can be spatially amplified (Briggs et al. 2013). Therefore, it is essential to maintain the high spatial heterogeneity in these landscapes for euglossine species requirements. This can be achieved through agricultural practices that small coffee producers carry out on their properties, such as coffee crops interspersed by many land uses, such as agroforestry and pastures, preferentially pastures interspersed by shrub vegetation and scattered trees.
In this study, we observed rare species’ occurrences and expanded the geographical distribution of a rare orchid bee species for the Atlantic Forest hotspot. Furthermore, we showed that a high coffee cover (%) drives euglossine communities to lower richness and species abundance. These coffee areas have negatively affected even superabundant species known for higher tolerance to environmental disturbances. Our findings could be extrapolated to other monoculture crops without any floral resources for Euglossini bees (e.g., Milet-Pinheiro and Schlindwein 2005), especially in systems with low environmental heterogeneity. Because the forest patches are in private areas and surrounded by agricultural environments, we suggest increasing the connectivity of these forest patches as a reasonable management measure to maintain euglossine communities’ stables in such landscapes.
Data availability
Additional data is available in the supplementary material.
Code availability
Not applicable.
Change history
09 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13592-022-00961-2
References
Aguiar WM, Gaglianone MC (2008) Comunidade de abelhas Euglossina (Hymenoptera: Apidae) em remanescentes de mata estacional semidecidual sobre tabuleiro no estado do Rio de Janeiro. Neotrop Entomol 37(2):118–125. https://doi.org/10.1590/s1519-566x2008000200002
Aguiar WM, Gaglianone MC (2012) Euglossine bee communities in small forest fragments of the Atlantic Forest, Rio de Janeiro state, southeastern Brazil (Hymenoptera, Apidae). Rev Bras Entomol 56(2):210–219. https://doi.org/10.1590/s0085-56262012005000018
Aguiar WM, Melo GAR, Gaglianone MC (2014) Does forest phisiognomy affect the structure of orchid bee (Hymenoptera, Apidae, Euglossini) communities? A study in the Atlantic Forest of Rio de Janeiro state, Brazil. Sociobiology 61(1):68–77. https://doi.org/10.13102/sociobiology.v61i1
Aguiar WM, Sofia SH, Melo GAR, Gaglianone MC (2015) Changes in orchid bee communities across forest-agroecosystem boundaries in Brazilian Atlantic forest landscapes. Environ Entomol 44(6):1465–1471. https://doi.org/10.1093/ee/nvv130
Ahuatzin DA, González-Tokman D, Valenzuela-González JE, Escobar F, Ribeiro MC, Acosta JCL, Dáttilo W (2021) Sampling bias in multiscale ant diversity responses to landscape composition in a human-disturbed rainforest. Insectes Soc 69(1):47–58. https://doi.org/10.1007/s00040-021-00844-2
Allen L, Reeve R, Nousek-McGregor A, Villacampa J, MacLeod R (2019) Are orchid bees useful indicators of the impacts of human disturbance? Ecol Indic 103:745–755. https://doi.org/10.1016/j.ecolind.2019.02.046
Briggs HM, Perfecto I, Brosi BJ (2013) The role of the agricultural matrix: Coffee management and Euglossine bee (Hymenoptera: Apidae: Euglossini) communities in Southern Mexico. Environ Entomol 42(6):1210–1217
Brosi BJ (2009) The effects of forest fragmentation on euglossine bee communities (Hymenoptera: Apidae: Euglossini). Biol Conserv 142(2):414–423. https://doi.org/10.1016/j.biocon.2008.11.003
Cândido MEMB, Morato EF, Storck-Tonon D, Miranda PN, Vieira LJS (2018) Effects of fragments and landscape characteristics on the orchid bee richness (Apidae: Euglossini) in an urban matrix, southwestern Amazonia. J Insect Conserv 22:475–486. https://doi.org/10.1007/s10841-018-0075-7
Cariveau DP, Winfree R (2015) Causes of variation in wild bee responses to anthropogenic drivers. Curr Opin Insect Sci 10:104–109. https://doi.org/10.1016/j.cois.2015.05.004
Carneiro LS, Aguiar WM, Aguiar CML, Santos GMM (2018) Orchid bees (Hymenoptera: Apidae: Euglossini) in seasonally dry tropical forest (Caatinga) in Brazil. Sociobiology, 65(2):253–258. https://doi.org/10.13102/sociobiology.v65i2.2077
Carneiro LS, Aguiar WM, Priante CF, Ribeiro MC, Frantine-Silva W, Gaglianone MC (2021) The interplay between thematic resolution, forest cover, and heterogeneity for explaining Euglossini bees community in an agricultural landscape. Front Ecol Evol 9:628319. https://doi.org/10.3389/fevo.2021.628319
Carneiro LS, Ribeiro MC, Aguiar WM, Priante CF, Frantine-Silve W, Gaglianone MC (2022) Orchid bees respond to landscape composition differently depending on the multiscale approach. Landsc Ecol. https://doi.org/10.1007/s10980-022-01442-8
Conab: Companhia Nacional do Abastecimento (2021) Boletim de Café- Setembro 2021 https://www.conab.gov.br/info-agro/safras/cafe. Accessed on 01 Oct 21
Cortopassi-Laurino M, Zillikens A, Steiner J (2009) Pollen sources of the orchid bee Euglossa annectans Dressler, 1982 (Hymenoptera, Apidae, Euglossini) analyzed from larval provisions. Gen Mol Res 8(2):546–556
Dicks LV, Breeze TD, Ngo HT, Senapathi D, An J, Aizen MA et al (2021) A global-scale expert assessment of drivers and risks associated with pollinator decline. Nat Ecol Evol 5(10):1453–1461. https://doi.org/10.1038/s41559-021-01534-9
Driscoll DA, Banks SC, Barton PS, Lindenmayer DB, Smith AL (2013) Conceptual domain of the matrix in fragmented landscapes. Trends Ecol Evol 28(10):605–613. https://doi.org/10.1016/j.tree.2013.06.010
Fahrig L (2017) Ecological responses to habitat fragmentation per se. Annu Rev Ecol Evol S 48(1):1–23. https://doi.org/10.1146/annurev-ecolsys-110316-022612
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 16(3):265–280. https://doi.org/10.1111/j.1466-8238.2007.00287.x
Gaston KJ (1994) What is rarity? In: Gaston KJ (ed) Rarity. Springer, Dordrecht, pp 1–21
Gestich CC, Arroyo-Rodríguez V, Ribeiro MC, da Cunha RGT, Setz EZF (2018) Unraveling the scales of effect of landscape structure on primate species richness and density of titi monkeys (Callicebus nigrifrons). Ecol Res 34(1):150–159. https://doi.org/10.1111/1440-1703.1009
Gonçalves RB, Faria LRR (2021) In euglossine we trust as ecological indicators: a reply to Añino et al. (2019). Sociobiology, 68(1):e4610. https://doi.org/10.13102/sociobiology.v68i1.4610
Hammer Ø, Harper DAT, Ryan PD (2001) PAST-palaeontological statistics, ver. 1.89. Palaeontol Electron 4:1–9
Hedström I, Denzel A, Owens G (2006a) Orchid bees as bio-indicators for organic coffee farms in Costa Rica: Does farm size affect their abundance? Rev Biol Trop 54(3):965–969
Hedström I, Harris J, Fergus K (2006b) Euglossine bees as potential bio-indicators of coffee farms: Does forest access, on a seasonal basis, affect abundance? Rev Biol Trop 54(4):1189–1195
Hesselbarth MHK, Sciaini M, With KA, Wiegand K, Nowosad J (2019) landscapemetrics: an open-source R tool to calculate landscape metrics. Ecography 42:1648–1657. https://doi.org/10.1111/ecog.04617
Hipólito J, Boscolo D, Viana BF (2018) Landscape and crop management strategies to conserve pollination services and increase yields in tropical coffee farms. Agri Ecosyst Environ 256:218–225. https://doi.org/10.1016/j.agee.2017.09.038
Huais PY (2018) multifit: an R function for multi-scale analysis in landscape ecology. Landsc Ecol 33:1023–1028. https://doi.org/10.1007/s10980-018-0657-5
International Coffee Organization (2021) Historical data on the global coffee trade https://ico.org/new_historical.asp. Accessed on 05 Oct 21
Janzen DH (1971) Euglossine bees as long-distance pollinators of tropical plants. Science 171(3967):203–205
Kremen C, Williams NM, Aizen MA, Gemmill-Herren B, LeBuhn G, Minckley R et al (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10(4):299–314. https://doi.org/10.1111/j.1461-0248.2007.01018.x
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Oxford, p 264
Martello F, Andriolli F, de Souza TB, Dodonov P, Ribeiro MC (2016) Edge and land use effects on dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) in Brazilian cerrado vegetation. J Insect Conserv 20(6):957–970. https://doi.org/10.1007/s10841-016-9928-0
Martello F, Andriolli FS, Medeiros HR, Barão T, Ribeiro MC (2022) Edge contrast modulates ant community responses to edge distance in agricultural landscapes. Agric for Entomol. https://doi.org/10.1111/afe.12492
Martins DC, Albuquerque PMC, Silva FS, Rebêlo JMM (2018) Orchid bees (Apidae: Euglossini) in Cerrado remnants in northeast Brazil. J Nat Hist 52(11–12):627–644. https://doi.org/10.1080/00222933.2018.1444210
McGarigal K (2015) FRAGSTATS help. University of Massachusetts, Amherst
Medeiros HR, Martello F, Metzger JP, Harper KA, Mengual X, Righi CA, Ribeiro MC (2021) Landscape composition regulates the spillover of beneficial insects between forest remnants and adjacent coffee plantations. Perspect Ecol Conserv. https://doi.org/10.1016/j.pecon.2021.11.003
Medeiros RLS, Aguiar WM, Aguiar CML, Borges IGM (2017) The orchid bee communities in different phytophysiognomies in the Atlantic Forest: from lowland to montane rainforests. Sociobiology, 64(2):182–190. https://doi.org/10.13102/sociobiology.v64i2.1348
Miguet P, Jackson HB, Jackson ND, Martin AE, Fahrig L (2016) What determines the spatial extent of landscape effects on species? Landsc Ecol 31:1177–1194. https://doi.org/10.1007/s10980-015-0314-1
Milet-Pinheiro P, Schlindwein C (2005) Do euglossine males (Apidae, Euglossini) leave tropical rainforest to collect fragrances in sugarcane monocultures? Rev Bras Zool 22(4):853–858
Moure JS, Melo GAR, Faria Jr LRR (2012) Euglossini Latreille, 1802, in: Moure JS, Urban D, Melo GAR (Eds) Catalogue of bees (Hymenoptera, Apoidea) in the Neotropical region - online version http://www.moure.cria.org.br/catalogue/. Accessed on 27 Mar 22
Nemésio A (2009) Orchid bees (Hymenoptera: Apidae) of the Brazilian Atlantic Forest. Zootaxa 2041:1–242. https://doi.org/10.11646/zootaxa.2041.1.1
Nemésio A, Engel MS (2012) Three new cryptic species of Euglossa from Brazil (Hymenoptera, Apidae). ZooKeys 222:47–68. https://doi.org/10.3897/zookeys.222.3382
Nemésio A, Silveira FA (2010) Forest fragments with larger core areas better sustain diverse orchid bee faunas (Hymenoptera: Apidae: Euglossina). Neotrop Entomol 39(4):555–561. https://doi.org/10.1590/s1519-566x2010000400014
Neves EL, Viana BF (1997) Inventário da fauna de Euglossinae (Hymenoptera, Apidae) do baixo sul da Bahia. Brasil Rev Bras Zool 14(4):831–837
Opedal ØH, Martins AA, Marjakangas EL (2020) A database and synthesis of euglossine bee assemblages collected at fragrance baits. Apidologie 51:519–530. https://doi.org/10.1007/s13592-02000739-4
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ramalho AV, Gaglianone MC, Oliveira ML (2009) Comunidades de abelhas Euglossina (Hymenoptera, Apidae) em fragmentos de Mata Atlântica no Sudeste do Brasil. Rev Bras Entomol 53(1):95–101
Rocha-Filho LC, Krug C, Silva CI, Garófalo CA (2012) Floral resources used by Euglossini bees (Hymenoptera: Apidae) in coastal ecosystems of the Atlantic Forest. Psyche 1–13. https://doi.org/10.1155/2012/934951
Rocha-Filho LC, Montagnana PC, Boscolo D, Garófalo CA (2020) Species turnover and low stability in a community of euglossine bees (Hymenoptera: Apidae) sampled within 28 years in an urban forest fragment. Apidologie 51(6):921–934. https://doi.org/10.1007/s13592-020-00772-3
Roubik DW, Hanson PE (2004) Orchid bees of tropical America: biology and field guide. INBio Press, Heredia
Silva DP, De Marco P (2014) No evidence of habitat loss affecting the orchid bees Eulaema nigrita Lepeletier and Eufriesea auriceps Friese (Apidae: Euglossini) in the Brazilian Cerrado Savanna. Neotrop Entomol 43(6):509–518. https://doi.org/10.1007/s13744-014-0244
Silveira GC (2014) Estrutura de comunidades de abelhas Euglossina (Hymenoptera; Apidae) e análise da distribuição em Florestas Estacionais Semideciduais e em paisagens fragmentadas no Sudeste do Brasil. Dissertation, Universidade Estadual do Norte Fluminense
Siqueira T, Bini LM, Roque FO, Marques Couceiro SR, Trivinho-Strixino S, Cottenie K (2012) Common and rare species respond to similar niche processes in macroinvertebrate metacommunities. Ecography 35:183–192. https://doi.org/10.1111/j.1600-0587.2011.06875.x
Sobreiro AI, Peres LLS, Boff S, Henrique JA, Alves Junior VV (2019) Continuous micro-environments associated orchid bees benefit from an Atlantic Forest remnant, Paraná state, Brazil. Sociobiology, 66(2):293–305. https://doi.org/10.13102/sociobiology.v66i2.3443
Sofia SH, Suzuki KM (2004) Comunidades de machos de abelhas Euglossina (Hymenoptera: Apidae) em fragmentos florestais no Sul do Brasil. Neotrop Entomol 33(6):693–702
Sousa FG, Santos JS, Martello F, Diniz MF, Bergamini LL, Ribeiro MC, Collevatti RG, Silva DP (2022) Natural habitat cover and fragmentation per se influence orchid-bee species richness in agricultural landscapes in the Brazilian Cerrado. Apidologie 53:20. https://doi.org/10.1007/s13592-022-00925-6
Tonhasca A, Blackmer JL, Albuquerque GS (2002) Abundance and diversity of Euglossine bees in the fragmented landscape of the Brazilian Atlantic Forest1. Biotropica 34(3):416–422. https://doi.org/10.1111/j.1744-7429.2002.tb00555.x
Venables WN, Ripley BD (2002) Modern Applied Statistics with S. Statistics and Computing. Springer, New York
Wikelski M, Moxley J, Eaton-Mordas A, López-Uribe MM, Holland R, Moskowitz D, Roubik DW, Kays R (2010) Large-range movements of neotropical orchid bees observed via radio telemetry. PLoS ONE 5:e10738. https://doi.org/10.1371/journal.pone.0010738
Wolda H (1981) Similarity indices, sample size and diversity. Oecologia 50:296–302
Acknowledgements
We thank the Bee Ecology and Pollination Lab team (UENF) for field help; Camila Priante for help with spatial analyses; the land owners for authorizing bee sampling; and Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis-IBAMA for the permission to collect biological material.
Funding
This study was funded by CNPq/MCTIC/IBAMA/A.B.E.L.H.A. (project 400614/2018–9). LSC and WFS received scholarship from CAPES (processes 88887.339454/2019–00; 88882.314552/2019–01). LSC received scholarship from FAPERJ (process E-26/200.279/2021). MCG received financial support from CNPq (process 303894/2018–0) and FAPERJ (process 203.321/2017). MCR received financial support from FAPESP (processes 2013/50421–2; 2020/01779–5; 2021/08534–0; 2021/10195–0) and CNPq (processes 442147/2020–1; 402765/2021–4; 313016/2021–6). MCG, MCR, GARM, and SHS are research fellows from CNPq.
Author information
Authors and Affiliations
Contributions
LSC, MCG, WMA designed the study and hypothesis, and wrote the manuscript; SHS contributed to the data discussion; LSC, MCR, WFS analyzed the data; GARM confirmed the taxonomic identifications. The manuscript was revised and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval
The bee sampling was authorized by Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis-IBAMA (N° 71013–3).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Not applicable.
Additional information
Manuscript editor: Cedric Alaux
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carneiro, L.d., Frantine-Silva, W., de Aguiar, W.M. et al. Coffee cover surrounding forest patches negatively affect Euglossini bee communities. Apidologie 53, 42 (2022). https://doi.org/10.1007/s13592-022-00952-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-022-00952-3