Abstract
Habitat loss and fragmentation in the human‐modified landscape affect bee assemblages by reducing the available natural resources. The life history features and adaptations of bees to environmental conditions affect how they perceive and interact with landscape structure. Therefore, it is crucial to quantify the magnitude of the effects of the landscape structure on this group at multiscale. We address the relative contribution of landscape structure in explaining the abundance of orchid bees, richness, and diversity in landscapes of Brazilian Cerrado. We mapped the surrounding landscape around 18 focal patches and sampled male orchid bees attracted by chemical baits. We used VIF and generalized linear models (GLM) to evaluate the effects of landscape structure (composition and configuration) at different spatial scales. We sampled 658 euglossine bees from five genera and 12 species. The savanna cover, forest cover, and fragmentation per se (i.e., the number of patches) were the best predictors of the richness of euglossine bees. The abundance of bees was explained by the savanna cover. The increase in natural habitat area and the reduction in landscape fragmentation favoring the assemblages of orchid bees are supported by our results. Savanna cover also modulates the abundance of euglossine bees, confirming that the habitat amount is essential for maintaining the populations of these bees. Our results reinforce the importance of habitat loss and fragmentation effects on the richness, abundance, and composition of euglossine bees, particularly in the naturally heterogeneous landscapes.
Similar content being viewed by others
1 Introduction
Landscape changes caused by natural area conversion for human activities jeopardize biodiversity and severely impact ecosystem structure and stability (Fahrig 2003). The decline of wild populations (Brow and Paxton 2009) and dispersal of individuals and migration rates may be caused by agricultural activities and other human‐induced changes in land cover via habitat loss and degradation. In such a scenario, a decrease in genetic diversity, along with a loss of ecosystem services, may also occur (Potts et al. 2016). Pollination is one of the vital ecosystem services affected by habitat loss in the Anthropocene (Dicks et al. 2021). The global decline in bee species richness and abundance has been of significant concern (Brown and Paxton 2009; Goulson et al. 2015) because of food security (Klein et al. 2007; FAO 2019) and the overall decline in plant diversity (Potts et al. 2010, 2016; Lundgren et al. 2016). Insects, especially bees, are the primary pollinators of most crops and native plants and are essential for production in ~ 75% of all crops used for human food (Klein et al. 2007; Ollerton et al. 2011).
Reciprocally, bees rely entirely on flowering plants for food, and a higher plant species richness is correlated with a higher number of bee pollinator species (Ebeling et al. 2008; Kaluza et al. 2018). Worldwide, agricultural expansion and intensification lead to natural vegetation loss and fragmentation, causing global bee declines. Therefore, the amount of habitat and structure of landscapes have crucial roles in conserving these insects (e.g., Brown and Paxton 2009; Winfree et al. 2009; Goulson et al. 2015; Cameron and Sadd 2020). For instance, while habitat loss and fragmentation decrease bee richness and abundance due to the reduction in floral and nesting resources (Brosi et al. 2008, 2009; Viana et al. 2012; Potts et al. 2010, Coswosk et al. 2018), natural habitats can buffer the harmful effects of pesticides (Park et al. 2015), climate change (Giannini et al. 2017; Papanikolaou et al. 2017), toxic metals (Barbosa et al. 2021), and invasive alien species (Garibaldi et al. 2021) on bee communities. Agricultural landscape heterogeneity and configuration may also increase the diversity of bees (e.g., Hass et al. 2018; Miljanic et al. 2019), providing different resources (Fahrig et al. 2011). However, in intensive farming, the abundance of bees may be reduced due to land use management and higher crop cover in the landscape (Hass et al. 2018). Additionally, the response of bees to anthropogenic disturbances may be related to life-history traits (Brosi et al. 2008; Williams et al. 2010). For instance, bee species nesting above the ground are more affected by habitat loss, isolation, and intensive farming than those nesting below the ground (Williams et al. 2010; Ferreira et al. 2015). Moreover, the response of bees to landscape features may be scale-dependent (Medeiros et al. 2019b), i.e., local and regional variation in landscape metrics may affect different components of community diversity and different taxonomic groups (Steffan-Dewenter et al. 2002; Ferreira et al. 2015; Montagnana et al. 2021). The importance of the α-diversity of pollinators (i.e., species richness and abundance) as a driver of ecosystem service delivery is well-demonstrated in various settings (e.g., pollination, pest control, water quality, disease control; Duarte et al. 2018). Therefore, a better understanding of how bee communities respond to landscape structure features at different scales is critical for reducing the anthropogenic disturbances’ impacts on the diversity and ecosystem services provided by this group (Winfree et al. 2011; Viana et al. 2012; Duarte et al. 2018).
In Brazil, at least 144 bee species have been associated with the 23 most important crops, including the critical commodity soybean, and 56% of them are solitary bees (Giannini et al. 2020). Thus, the decline in bee diversity and abundance may have direct economic impacts in Brazil, estimated at ~ 4.86 to 14.56 billion dollars/year for the 29 major food crops, representing 6.46–19.36% of the Brazilian GDP (Novais et al. 2018). In the last decades, deforestation rates as high as 1% per year have been caused by agriculture and livestock expansion in the Brazilian Cerrado, leading to a loss of 50% of its original area (Sano et al. 2010; Alencar et al. 2020). The Brazilian Cerrado is naturally composed of a vegetative mosaic of savanna-like formations and forest forms (Ribeiro and Walter 1998). The Brazilian Cerrado is the most biodiverse and threatened savanna globally (Silva and Bates 2002). Because of Cerrado degradation, plant species may be at higher extinction risk (Strassburg et al. 2017; Velazco et al. 2019), and the addition of habitat loss, pesticide use, and farming management may also threaten bee species (Potts et al. 2016).
Orchid bees (Apidae: Euglossini) pollinate hundreds of native and cultivated plant species (e.g., Dressler 1982; Roubik 1992; Giannini et al. 2015; Silveira et al. 2015). Many Euglossini species have a remarkable flying capacity and may travel long distances in continuous forests (Janzen 1971; Pokorny et al. 2015; Wikelski et al. 2010), but small-size species are forest dependent, rarely leaving forest fragments (Milet-Pinheiro and Schlindwein 2005). Most studies investigating the influence of landscape structure on orchid bees have been carried out in the rainforest (e.g., Powell and Powell 1987; Tonhasca et al. 2002; Storck-Tonon et al. 2013, Storck-Tonon and Peres 2017; Rosa et al. 2015; Cândido et al. 2018). Only a handful of studies have been carried out in the Brazilian Cerrado (e.g., Silva and De Marco 2014; Moreira et al. 2017; Silva et al. 2017).
Here, we analyze the effects of landscape composition and configuration on the α-diversity of Euglossini communities in the Brazilian Cerrado. Our study comprises landscapes in intensive farming systems and a protected area, and we assessed the landscape effects using a multiscale approach (Jackson and Fahrig 2015; Gestich et al. 2019). We expect that landscapes with higher habitat cover and heterogeneity to harbor higher α-diversity, i.e., abundance, species richness, and diversity of bees (see our predictions in Fig. 1). On the other hand, landscapes with higher agriculture and pasture cover, with fewer habitat patches and a higher patch shape index (PSI) (more irregular patches), have lower α-diversity.
2 Material and methods
2.1 Study area and sampling sites
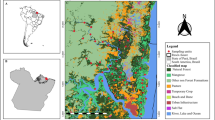
We carried out the study in the long-term ecological research (LTER) project, the COFA LTER project (Functional Connectivity in Agricultural Landscapes) in Central-Western Brazil (Fig. 2A), one of the most important agribusiness regions in the country. The landscape comprises a mosaic of agriculture, mainly soybean, corn, pasture, Cerrado savanna remnants, seasonally dry and riparian forests (Fig. 2B), and a protected area, the National Forest of Silvânia with 487.4 ha (Santos et al. 2021; Fig. 2B).
Spatial distribution of the 18 sampling sites of Euglossini bees. (A) The Brazilian Cerrado distribution (gray) depicting the study area (B) the COFA LTER study area with land cover composition and the 18 sampling sites (C) multiscale sampling design used to calculate the landscape metrics. Buffer radii sizes are 500, 750, and 1000 m. Land cover colors correspond to different categories according to the figure legends.
We mapped the land cover by visual digitalization and manual classification using high-resolution images freely available at the Geographic Information System QGIS (QGIS Development Team 2017) using the Open Layer Plugin at a scale of 1:5000 m. We mapped 11 land cover types (Fig. 2B): savanna, seasonally dry forest, riparian forest, wetlands, agriculture (soy/corn), pasture, urban areas, rural buildings, roads, train rails, and water. We performed field inspections to ensure high map accuracy. For landscape analyses, we merged similar land cover types due to their spatial distribution and the low number of some cover types in the landscape, such as riparian and seasonally dry forests (forest, hereafter), savanna and wetlands (savanna, hereafter), and urban areas, rural buildings, roads, and train rails (urban area, hereafter).
We selected 18 landscapes representing landscape composition and configuration gradients using a buffer size of 1000 m around the sampling sites. Therefore, these landscapes varied their forest and savanna cover, compositional heterogeneity (i.e., Shannon diversity index), number of forest patches, and patch shape (see metric calculations below).
2.2 Bee community sampling
Within each of the 18 landscapes, we selected one site in a forest remnant (Fig. 2B, Table S1) to sample the orchid bee community. We installed six sampling stations 50 m apart from each other at each sampling site. Each sampling station comprised six scent traps installed 1.5 m above ground and 3 m apart (see details in Appendix Figure S1). We built scent traps with 2-L plastic polyethylene terephthalate (PET) bottles, with three funnel inlets on their sides covered with sandpaper to increase friction and facilitate the entrance of the bees. Inside the base of the bottles, we soaked a cotton swab with chemical baits and soapy water. We used six aromatic essences: eucalyptol, methyl salicylate, vanillin, eugenol, methyl cinnamate, and benzyl acetate. At each sampling site, traps remained in the field for 24 h in two field surveys during the rainy season from December 2017 to March 2018. All bees were collected and identified at the species level following the accepted names from the Catalogue of Bees (Hymenoptera, Apoidea) in the Neotropical Region (Bembé 2007; Ferrari and Melo 2014; Hinojosa-Díaz and Engel 2014; Moure et al. 2007; Nemésio 2009; Rebelo and Moure 1995; Roubik 2004). We deposited all specimens in the Zoology Collection of the Universidade Federal de Goiás (Goiânia, Goiás, Brazil).
We estimated α-diversity using abundance, species richness, and diversity for each sampling site. We obtained the number of individuals per species (species abundance), the total number of species, and the individuals sampled. We estimated species richness using the Jackknife method (Heltshe and Forrester, 1983) and the Shannon–Wiener diversity index (H′) using the vegan package (Oksanen et al. 2017) in R version 3.6.1. (R Core Team 2019).
We tested spatial autocorrelation in our community data using Moran’s I test implemented in ape package (Paradis and Schliep 2019) in R version 3.6.1. We found no significant spatial autocorrelation (Table S2) for species richness (r = −0.092; p = 0.432), abundance (r = −0.014, p = 0.282), nor Shannon–Wiener diversity (r = −0.091, p = 0.447).
2.3 Multiscale landscape metrics
We calculated the landscape composition and configuration to analyze the effects of habitat loss and fragmentation on orchid-bee communities. We calculated the metrics at three spatial scales, defined by concentric buffers of 500 m, 750 m, and 1000 m radii around each sampling site. We chose these radii buffers considering the flight range of the orchid bees according to the diversity of the group based on different estimations (e.g., homing, foraging, and communication distance, and range of body size; Borges et al. 2020) and the maximum distance to avoid overlap among landscapes.
We calculated habitat amount as the percentage of two natural vegetation covers (savanna and forest) since different habitats are associated with different floral and nest resource availability (Moreira et al. 2017). We also calculated the proportion of agriculture (mainly soybean and corn crops) and pasture in the landscape. We calculated landscape compositional heterogeneity using the Shannon index (SHDI, McGarigal et al. 2012), considering all land cover categories.
We measured landscape fragmentation or subdivision by calculating the number of forest (NP_forest) and savanna (NP_savanna) patches, as well as PSI (McGarigal et al. 2012; Fahrig 2019). With PSI, the complexity of a patch shape compared to a standard shape (square) of the same size is measured (McGarigal et al. 2012). We calculated the PSI, including savannas and forest patches, and obtained the mean shape index for each landscape spatial scale, ignoring the zero values. Landscapes covered by a single patch had a shape index = 1, assuming the patch as regular. We calculated the landscape metrics using the landscapemetrics package (Hesselbarth et al. 2019) implemented in R version 3.6.1. (R Core Team 2019).
2.4 Data analyses
To analyze the effects of landscape composition and configuration in α-diversity in the orchid-bee community, we first selected the scale of effect (Jackson and Fahrig 2015) for each response variable and landscape metrics. We performed a multiscale test of independence for multivariate vectors using the multifit function (Huais 2018) implemented in R version 3.6.1. We used R2 as a measure of the relationship’s strength to choose the scale for each response variable (Table S3).
We estimated the variance inflation factor (VIF) to assess collinearity among landscape metrics selected by the effect scale (Tables S4–S6). We used the jtools package (Long 2020) available in R with a stepwise approach, removing models with predictors with high collinearity (VIF > 5.0, Zuur et al. 2009).
Finally, to analyze landscape effects on community α-diversity, we used the selected explanatory variables by multicollinearity analysis (Table I) to perform Generalized Linear Models (GLM) using R version 3.6.1. We generated nine univariate models for each response variable: one for each landscape predictor and a null model, including only the intercept, representing the absence of specific landscape processes. We used Gaussian distribution to estimate species richness and diversity models and negative binomial distribution for abundance models. We selected the best predictive model using AIC (Akaike’s information criterion; Burnham and Andersen 2002) with the bbmle package (Bolker 2009; Bolker and R Development Core team 2020) in R version 3.6.1. We estimated AIC corrected for small sample sizes (AICc), each difference of the model, and the best model (ΔAICc) for model ranking. We also estimated Akaike’s weight of evidence (wAICc) as the relative contribution of each model to explain the observed pattern. Models with ΔAICc < 4.0 were considered equally likely (Zuur et al. 2009).
3 Results
3.1 The α-diversity of the bees community
We sampled 658 Euglossini bees from 12 species and five genera (Table II). Most specimens were Eulaema (n = 401; 60.9%), followed by Euglossa (30.4%), Eufriesea (7.9%), Exaerete (n = 2; 0.3%), and one specimen of the monospecific genus Aglae. Eulaema nigrita Lepeletier (1841) was the most abundant, with 349 (53.0%) individuals sampled pooling all sites, followed by Euglossa townsendi Cockerell (1922), with 102 (15.5%) specimens sampled, and Euglossa imperialis Cockrell (1922) (n = 68, 10.3%). The most frequent species were Eul. nigrita, found in all 18 sites, and Eug. townsendi and Eulaema cingulata (Fabricius 1804), which occurred in 16 out of 18 sampling sites.
The estimated species richness ranged from 2.0 to 10.5, and the Shannon–Wiener diversity index ranged from 0.517 to 1.804 (Table S1). Sites 7 and 9, within the protected area (Flona Silvânia, Fig. 2B), showed the highest estimated species richness (10.500 and 8.833) and diversity (1.968 and 1.951) (Table S1).
3.2 Landscape effects on bee diversity
Bee species abundance, richness, and diversity had a similar scale of effect (i.e., higher values of R2), 500 m, for most variables (Tables I and S3). Forest cover (%) affected the bee community at the 1000-m spatial scale for the three variables (Table I, Table S3). The number of savanna patches and PSI had a different scale of effects on the three response variables (Table I, Table S3). We excluded agriculture cover (%) from our models because it had high collinearity (VIF > 5.0) with all the other response variables (Tables S4 to S6).
Species richness was explained by three models: the percentage of savanna cover at 500-m spatial scale (wAICc = 0.530, Table III), the number of forest patches at 500-m spatial scale (wAICc = 0.185, Table III), and the proportion of forest cover at 1000-m spatial scale (wAICc = 0.119, Table III). Both the proportion of savanna cover in the landscape (Fig. 3A) and forest cover increased bee richness (Fig. 3B), while the number of forest patches decreased it (Fig. 3C). The savanna cover at 500-m spatial scale positively influenced the variation in Euglosini species abundance among sampling sites (wAICc = 0.819, Table III. Figure 3D). Although several landscape metrics explained Shannon diversity variation, the null model was equally likely (Table III). The pasture cover in the landscape, the number of savanna patches, and the PSI of forest and savanna could not explain any response variables (Table S9).
Relationship between Euglossini α-diversity and landscape composition and configuration based on 18 sampling sites in the Brazilian Cerrado. (A) Species richness and proportion of savanna cover in the landscape at 500-m spatial scale. (B) Species richness and proportion of forest cover in the landscape at a 1000-m spatial scale. (C) Species richness and the number of forest patches in the landscape at the 500-m spatial scale. (D) Abundance and proportion of savanna cover in the landscape at the 500-m spatial scale. The solid line represents the fitted model, and the shaded area is the 95% confidence interval.
4 Discussion
Our findings show that the α-diversity of the Euglossini communities in the Brazilian Cerrado is affected by landscape composition and configuration. Landscape configuration, measured by the number of forest patches, affected species richness on a small spatial scale (500 m), while landscape composition affected both small (500 m) and large (1000 m) spatial scales. The abundance of bees was also influenced by landscape composition on a small spatial scale. However, we analyzed scales up to 1000 m, suggesting that local landscape features affecting ecological processes may determine the α-diversity in Euglossini bees at the regional scale. Forest cover also explains bee species richness on a small spatial scale (500 m) in coffee plantations in the Atlantic Forest, Brazil (Medeiros et al. 2019a). In these coffee agroecosystems, wasp richness and abundance were also best explained by forest cover on a small scale (750 m and 500 m, respectively).
Although most of the species we sampled here are habitat and pollen-resource generalists, such as Eul. nigrita, Eug. townsendi, Eug. imperialis, Eul. cingulata, and Eufriesea auriceps, we found strong evidence of negative effects of either savanna or forest loss in species richness and abundance. Habitat generalists Euglossini, such as some Eulaema species (e.g., Eul. nigrita), may have a higher abundance in fragmented landscapes in different ecosystems (e.g., Powell and Powell 1987; Otero and Sandino 2003; Brosi 2009; Silva et al. 2014), showing high resilience to habitat loss and fragmentation. Mostly, generalists Euglossini have a large body size and a remarkable flying capability (e.g., Wikelski et al. 2010; Pokorny et al. 2015). Still, small body-sized generalist species may also fly large distances between habitat remnants (e.g., Tonhasca et al. 2003; Milet-Pinheiro and Schlindwein 2005), contributing to such fragmentation resilience. However, the negative effects of landscape changes in bee communities have been shown in several studies, as we found here. For instance, visitation rates to flowers and the abundance of Euglossini bees decrease with reduced fragment size in Amazonia (e.g., Powell and Powell 1987). In agricultural landscapes, the abundance of above-ground nesting bees decreases with habitat loss and isolation (Williams et al. 2010), and species composition changes between forest and pasture, with no orchid bees sampled in pastures (Brosi et al. 2008). The abundance of Euglossini bees also has a positive relationship with fragment size (Brosi 2009), percentage of savanna, and compositional heterogeneity in agroecosystems (Briggs et al. 2013; Moreira et al. 2017). Orchid bees show a unique reproductive behavior, where males forage for fragrances in external sources, mainly orchids and flowers from other families, as well as resins and fungi, which are then used to attract mates (Dressler 1982). Despite their high mobility to explore landscapes, the degree of specialization in fragrance sources may also play a role in their response to landscape configuration. Such a specialization degree is not well known yet (Mitko et al. 2016; Hetherington-Rauth and Ramírez 2016) and deserves more investigation in future studies. It is important to note that orchid-bee species in the Cerrado are usually more habitat generalist than those from forested areas, composing a completely different species pool compared with rainforests (Silveira et al. 2015). Thus, we expected to sample more generalists than specialist species in Cerrado.
Despite the recent intensive farming in the studied area (~ 60 years), we found strong evidence of adverse effects of habitat loss and fragmentation in the Euglossini bee communities in Cerrado. These bees are generally open-area generalists. Indeed, the effects of habitat loss and fragmentation on bees are more pronounced in highly disturbed landscapes with very low habitat amount (Winfree et al. 2009). Our landscapes are embedded in a highly intensive farming system with tiny patches of forest and savanna, except by a large savanna area (sites 7 and 9, Fig. 2), which is the protected area Silvânia National Forest (see Santos et al. 2021). Landscapes in the protected area had higher species richness and abundance, higher habitat cover, and fewer forest patches than the other landscapes. Habitat cover may influence resource availability for bees, such as floral, nesting, and fragrance resources, and may strongly limit species richness and abundance.
Intensive agricultural practices may directly and adversely affect bees, such as the impacts of pesticides (Cresswell 2011; Gill et al. 2012). This is especially relevant for those species that use crop plants as nectar and pollen resources, as is the case for several Euglossa species (Giannini et al. 2015). The highly intensive farming and habitat loss in our study area and the differences in spatial scale may explain the divergence of our results from other studies that did not find any habitat loss effect upon the abundance of the Euglossini Eul. nigrita and Euf. auriceps. Silva and De Marco (2014) studied landscapes on a broader geographic scale, where macroecological processes may be more important than ecological processes at the local landscape level (Wiens 1989). Furthermore, in this study, additional metrics of orchid-bee diversity were explored.
In conclusion, we show that despite the recent agriculture expansion (~ 60 years), agriculture intensification leading to fragmentation and habitat loss affects Euglossini bees at a landscape scale. Due to habitat loss, the decrease in plant species richness and abundance in the studied area (Santos et al., 2022) may have affected the foraging and nesting resources for orchid bees, causing a decline in species richness and abundance. This effect is expected to be even more pronounced for orchid bees than many bee groups, considering that their orchid host plants are especially vulnerable to habitat loss and fragmentation in other regions (Hernández-Pérez and Solano 2015; Hundera et al. 2013). Once many orchid species are highly specialized in their Euglossini pollinators (Ramírez et al. 2011), habitat loss may lead to co-extinctions and other synergistic deleterious effects for this particular system.
Euglossini bees are more diverse in forested environments (Nemésio and Silveira 2007), essential habitats, and ecological corridor elements (Moura and Schlindwein 2009; Silva et al. 2013). In the COFA LTER study area, most riparian and seasonally dry forest patches are linear or irregular. Thus, they have less core area and higher edge effects (Santos et al., 2022). This can impact habitat specialist species, increase population size variability, and decrease the probability of population persistence (Didham 2010). The nesting and foraging behaviors of orchid bees may be negatively affected by environmental stress, such as high temperatures and low humidity (Vilhena et al. 2017). On the other hand, such effects may be softened in fragments with a higher amount of core area and a more negligible edge effect (Nemésio and Silveira 2006, 2010; Cândido et al. 2018). Thus, the maintenance of forest patches in agricultural landscapes is critical to minimize habitat loss and fragmentation in Euglossini bees. Even though Euglossini bees may disperse over long distances and in the agroecosystem matrix, resources for many species may be provided by forest and savannas restoration. Therefore, the richness and abundance of Euglossini bees may be increased, maintaining the ecosystem services provided by them, which are essential pollinators for both crops and native plants.
Availability of data and material
The datasets generated during the field samplings or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Alencar, A., Shimbo, J.Z., Lenti, F., Marques, C.B., Zimbres, B., et al. (2020) Mapping three decades of changes in the brazilian savanna native vegetation using landsat data processed in the google earth engine platform. Remote Sens. 12, 924. https://doi.org/10.3390/rs12060924
Barbosa, M.M., Fernandes, A.C.C., Alves, R.S.C., Alves, D.A., Junior, F.B., Batista, B.L., Ribeiro, M.C., Carneiro, M.F.H. (2021) Effects of native forest and human-modified land covers on the accumulation of toxic metals and metalloids in the tropical bee Tetragonisca angustula. Ecotoxicol. Environ. Saf. 215, 112147
Bembé, B. (2007) Revision der Euglossa cordata-Gruppe und Untersuchungen zur Funktionsmorphologie und Faunistik der Euglossini (Hymenoptera, Apidae). Entomofauna Suppl. 14, 1–146
Bolker, B., R Development Core Team (2020) bbmle: tools for general maximum likelihood estimation. R package version 1.0.23.1. https://CRAN.R-project.org/package=bbmle. Accessed 1 Jan 2019
Bolker, B.M. (2009) Ecological models and data in R. Princeton, NJ: Princeton University Press
Borges, R.C., Padovani, K., Imperatriz-Fonseca, V.L. et al. (2020) A dataset of multi-functional ecological traits of Brazilian bees. Sci Data 7, 120. https://doi.org/10.1038/s41597-020-0461-3
Briggs, H.M., Perfecto, I., Brosi, B.J. (2013) The role of the agricultural matrix: coffee management and euglossine bee (hymenoptera: apidae: euglossini) communities in Southern Mexico. Environ. Entomol. 42, 1210–1217. https://doi.org/10.1603/EN13087
Brosi, B.J. (2009) The effects of forest fragmentation on Euglossine bee communities (Hymenoptera: Apidae: Euglossini). Biol. Conserv. 142, 414–423
Brosi, B.J., Daily, G.C., Shih, T.M., Oviedo, F., Durán, G. (2008) The effects of forest fragmentation on bee communities in tropical countryside. J. Appl. Ecol. 45, 773–783
Brown, M.J.F., Paxton, R.J. (2009) The conservation of bees: a global perspective. Apidologie 40, 410–416
Burnham, K.P., Andersen, D.R. (2002) Model selection and multi-model inference. Springer, New York
Cameron, S.A., Sadd, B.M. (2020) Global trends in bumble bee health. Annu. Rev. Entomol. 65, 209–232
Cândido, M.E.M.B., Morato, E.F., Storck-Tonon, D., Miranda, P.N., Vieira, L.J.S. (2018) Effects of fragments and landscape characteristics on the orchid bee richness (Apidae: Euglossini) in an urban matrix, southwestern Amazonia. J. Insect Conserv. 22, 1-12. https://doi.org/10.1007/s10841-018-0075-7
Coswosk, J.A., Ferreira, R.A., Soares, E.D.G., Faria, L.R.R. (2018) Responses of euglossine bees (Hymenoptera, Apidae, Euglossina) to an edge-forest gradient in a large Tabuleiro forest remnant in eastern Brazil. Neotrop. Entomol. 47, 447–456
Cresswell, J.E. (2011) A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology 20, 149– 57
Dicks, L.V., Breeze, T.D., Ngo, H.T. et al. (2021) A global-scale expert assessment of drivers and risks associated with pollinator decline. Nat Ecol Evol 5, 1453–1461. https://doi.org/10.1038/s41559-021-01534-9
Didham, R.K. (2010) Ecological consequences of habitat fragmentation. Encyc. Life Sci. 61, 1–39. https://doi.org/10.1002/9780470015902.a0021904
Dressler, R.L. (1982) Biology of the orchid bees (Euglossini). Annu. Rev. Ecol. Evol. Syst. 13, 373–394. https://doi.org/10.1146/annurev.es.13.110182.002105
Duarte, G.T., Santos, P.M., Cornelissen, T.G., Ribeiro, M.C., Paglia, A.P. (2018) The effects of landscape patterns on ecosystem services: meta-analyses of landscape services. Landsc. Ecol. 33, 1247–1257. https://doi.org/10.1007/s10980-018-0673-5
Ebeling, A., Klein, A.M., Schumacher, J., Weisser, W.W., Tscharntke, T. (2008) How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos. 117, 1808–1815. https://doi.org/10.1111/j.1600-0706.2008.16819.x
Fahrig, L. (2003) Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics, 34, 487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Fahrig, L. (2019) Habitat fragmentation: A long and tangled tale. Global Ecol. Biogeogr. 28, 33–41
Fahrig, L., Baudry, J., Brotons, L., Burel, F.G., Crist, T.O., Fuller, R.J., Sirami, C., Siriwardena, G.M., Martin, J.L. (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 14, 101–112
FAO (2019) Declining bee populations pose threat to global food security and nutrition. Retrieved from http://www.fao.org/news/story/en/item/1194910/icode/. Accessed 1 Jan 2019
Ferrari, B., Melo, G.A.R. (2014) Deceiving colors: Recognition of color morphs as separate species in orchid bees is not supported by molecular evidence. Apidologie 45, 641-652
Ferreira, P.A., Boscolo, D., Carvalheiro, L.G., Biesmeijer, J.C., Rocha, P.L.B., Viana, B.F. (2015) Responses of bees to habitat loss in fragmented landscapes of Brazilian Atlantic Rainforest. Landsc. Ecol. 30(10), 2067–2078. https://doi.org/10.1007/s10980-015-0231-3
Garibaldi, L.A., Oddi, F.J., Miguez, F.E., Bartomeus, I., Orr, M.C., et al. (2021) Working landscapes need at least 20% native habitat. Conserv. Lett. 14, 12773
Gestich, C.C., Arroyo‐Rodríguez, V., Ribeiro, M.C., Cunha, R.G.T., Setz, E.Z.F. (2019) Unraveling the scales of effect of landscape structure on primate species richness and density of titi monkeys (Callicebus nigrifrons). Ecol. Res. 34, 150–159. https://doi.org/10.1111/1440-1703.1009
Giannini, T.C., Alves, D.A., Alves, R., Cordeiro, G.D., Campbell, A.J., Awade, M., Bento, J.M.S., Saraiva, A.M., Imperatriz-Fonseca, V.L. (2020) Unveiling the contribution of bee pollinators to Brazilian crops with implications for bee management. Apidologie 51, 406–421. https://doi.org/10.1007/s13592-019-00727-3
Giannini, T.C., Boff, S., Cordeiro, G.D., Cartolano-Jr, E.A., Veiga, A.K., Imperatriz-Fonseca, V.L., Saraiva, A.M. (2015) Crop pollinators in Brazil: a review of reported interactions. Apidologie 46, 209–223. https://doi.org/10.1007/s13592-014-0316-z
Giannini, T.C., Costa, W.F., Cordeiro, G.D., Imperatriz-Fonseca, V.L., Saraiva, A.M., Biesmeijer, J., Garibaldi, L.A. (2017) Projected climate change threatens pollinators and crop production in Brazil. PLoS ONE. 12, 0182274
Gill, R.J., Ramos-Rodriguez, O., Raine, N.E. (2012) Combined pesticide exposure severely affects individual and colony-level traits in bees. Nature 491, 105–08
Goulson, D., Nicholls, E., Botías, C., Rotheray, E.L. (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957
Hass, A.L., Kormann, U.G., Tscharntke, T., Clough, Y., Baillod, A.B., Sirami, C., Fahrig, L., Martin, J.L., Baudry, J., Bertrand, C., Bosch, J., Brotons, L., Bure, F., Georges, R., Giralt, D., Marcos-García, M., Ricarte, A., Siriwardena, G., Batáry, P. (2018) Landscape configurational heterogeneity by small-scale agriculture, not crop diversity, maintains pollinators and plant reproduction in western Europe. Proc. R. Soc. B. 285(1872), 20172242. https://doi.org/10.1098/rspb.2017.2242
Heltshe, J.F., Forrester, N.E. (1983) Estimating species richness using the jackknife procedure. Biometrics 39, 1–11
Hernández-Pérez, E., Solano, E. (2015) Effects of habitat fragmentation on the diversity of epiphytic orchids from a montane forest of southern Mexico. J. Trop. Ecol. 31(2), 103–115. https://doi.org/10.1017/S0266467414000662
Hesselbarth, M.H.K., Sciaini, M., With, K.A., Wiegand, K., Nowosad, J. (2019) Landscapemetrics: an open-source R tool to calculate landscape metrics. Ecography 42, 1648–1657. https://doi.org/10.1111/ecog.04617
Hetherington-Rauth, M.C., Ramírez, S.R. (2016) Evolution and diversity of floral scent chemistry in the euglossine bee-pollinated orchid genus Gongora. Ann. Bot. 118(1), 135–148. https://doi.org/10.1093/aob/mcw072
Hinojosa-Díaz, I.A. & Engel, M.S. (2014) Revision of the orchid bee subgenus Euglossella (Hymenoptera: Apidae), part II: The viridis and mandibularis species groups. Journal of Melittology. 36, 1–108
Huais, P.Y. (2018) multifit: an R function for multiscale analysis in landscape ecology. Landsc. Ecol. 33, 1023–1028. https://doi.org/10.1007/s10980-018-0657-5
Hundera, K., Aerts, R., Beenhouwer, M., Overtveld, K.V., Helsen, K., Muys, B., Honnay, O. (2013) Both forest fragmentation and coffee cultivation negatively affect epiphytic orchid diversity in Ethiopian moist evergreen Afromontane forests. Biol. Conserv. 159, 285–291. https://doi.org/10.1016/j.biocon.2012.10.029.
Jackson, H.B., Fahrig, L. (2015) Is research conducted at optimal scales? Global Ecol. Biogeogr. 24, 52–63. https://doi.org/10.1111/geb.12233
Janzen, D.H. (1971) Euglossine bees as long-distance pollinators of tropical plants. Science 171, 203–205
Kaluza, B.F., Wallace, H.M., Heard, T.A., Minden, V., Klein, A., Leonhardt, S.D. (2018) Social bees are fitter in more biodiverse environments. Sci. Rep. 8, 12353. https://doi.org/10.1038/s41598-018-30126-0
Klein, A.M., Vaissière, B.E., Cane, J.H., Steffan-Dewenter, I., Cunningham, S.A., Kremen, C., Tscharntke, T. (2007) Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B. 274, 303–13. https://doi.org/10.1098/rspb.2006.3721
Long, J.A. (2020) Jtools: analysis and presentation of social scientific data. R package version 2.1.0. https://cran.r-project.org/package=jtools. Accessed 1 Jan 2019
Lundgren, R., Totland, O., Lázaro, A. (2016) Experimental simulation of pollinator decline causes community‐wide reductions in seedling diversity and abundance. Ecology. 97, 1420–1430. https://doi.org/10.1890/15-0787.1
McGarigal, K., Cushman, S.A., Ene, E. (2012) FRAGSTATS v4: spatial pattern analysis program for categorical and continuous maps. In: Computer software program produced by the authors at the University of Massachusetts, Amherst
Medeiros. H.R., Grandinete, Y.C., Manning, P., Harper, K.A., Cutler, G.C., Tyedmers, P., Righi, C.A., Ribeiro, M.C. (2019a) Forest cover enhances natural enemy diversity and biological control services in Brazilian sun coffee plantations. Agron. Sustain. Dev. 39(50). https://doi.org/10.1007/s13593-019-0600-4
Medeiros, H.R., Martello, F., Almeida, E.A.B., Mengual, X., Harper, K.A., Grandinete, Y.C., Metzger, J.P., Righi, C.A., Ribeiro, M.C. (2019b). Landscape structure shapes the diversity of beneficial insects in coffee producing landscapes Biol. Conserv. 238, 108193. https://doi.org/10.1016/j.biocon.2019a.07.038
Milet-Pinheiro, P., Schlindwein, C. (2005). Do euglossine males (Apidae, Euglossini) leave tropical rainforest to collect fragrances in sugarcane monocultures? Rev. Bras. Zootec. 22(4), 853–858. https://doi.org/10.1590/S0101-81752005000400008
Miljanic, A.S., Loy, X., Gruenewald, D.L., Dobbs, E.K., Gottlieb, I.G.W., Fletcher, R.J., Brosi, B.J. (2019) Bee communities in forestry production landscapes: interactive effects of local-level management and landscape context. Landsc. Ecol. 34, 1015–1032. https://doi.org/10.1007/s10980-018-0651-y
Mitko, L., Weber, M.G., Ramirez, S.R., Hedenström, E., Wcislo, W.T., Eltz, T. (2016) Olfactory specialization for perfume collection in male orchid bees. J. Exp. Biol. 219, 1467–1475. https://doi.org/10.1242/jeb.136754
Montagnana, P.C., Alves, R.S., Garófalo, C.A., Ribeiro, M.C. (2021) Landscape heterogeneity and forest cover shape cavity-nesting hymenopteran communities in a multiscale perspective. Basic Appl. Ecol.
Moreira, E.F., Santos, R.L.S., Silveira, M.S., Boscolo, D. (2017) Influence of landscape structure on Euglossini composition in open vegetation environments. Biota Neotrop. 17, 1–7
Moura, D.C., Schlindwein, C. (2009) Mata Ciliar do Rio São Francisco como Biocorredor para Euglossini (Hymenoptera: Apidae) de Florestas Tropicais Úmidas. Neotrop. Entomol. 38, 281–284.
Moure, J.S., Melo, G.A.R., Faria, L.R.R. (2007) Euglossini Latreille, 1802. In: Moure, J. S., Urban, D., Melo, G. A. R. (Eds.) Catalogue of Bees (Hymenoptera, Apoidea) in the Neotropical Region. Curitiba, Sociedade Brasileira de Entomologia, 214–255
Nemésio, A. (2009) Orchid bees (Hymenoptera:Apidae) of the Brazilian Atlantic Forest. Zootaxa 2041, 1–242
Nemésio, A., Silveira, F.A. (2006) Edge effects on the orchid-bee fauna (Hymenoptera: Apidae) at a large remnant of Atlantic Rain Forest in southeastern Brazil. Neotrop. Entomol. 35(3), 313–323. https://doi.org/10.1590/S1519-566X2006000300004
Nemésio, A., Silveira, F.A. (2007) Diversity and distribution of orchid bees (Hymenoptera: Apidae) with a revised checklist of species. Neotrop. Entomol. 36, 874–888.
Nemésio, A., Silveira, F.A. (2010) Forest fragments with larger core areas better sustain diverse orchid bee faunas (Hymenoptera: Apidae: Euglossina). Neotrop. Entomol. 39(4), 555–561. https://doi.org/10.1590/S1519-566X2010000400014
Novais, S.M.A., Nunes, C.A., Santos, N.B., D`Amico, A.R., Fernandes, G.W., Quesada, M., Braga, R.F., Neves, A.C.O. (2018) Correction: Effects of a Possible Pollinator Crisis on Food Crop Production in Brazil. PLoS ONE, 13(5), e0197396. https://doi.org/10.1371/journal.pone.0197396
Oksanen, F.J., Blanchet, G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O'Hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H., Szoecs, E., Wagner, H. (2017) Vegan: Community Ecology Package. R package version 2.4–4
Ollerton, J., Winfree, R., Tarrant, S. (2011) How many flowering plants are pollinated by animals? Oikos 120, 321–326
Otero, J.T., Sandino, J.C. (2003) Capture rates of male euglossine bees across a human intervention gradient, Choco ́ region, Colombia. Biotropica 35, 520–529.
Papanikolaou, A.D., Kühn, I., Frenzel, M., Schweiger, O. (2017) Semi‐natural habitats mitigate the effects of temperature rise on wild bees. J Appl Ecol 54, 527-536
Paradis, E., Schliep, K. (2019) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528
Park, M.G., Blitzer, E.J., Gibbs, J., Losey, J.E., Danforth, B.N. (2015) Negative effects of pesticides on wild bee communities can be buffered by landscape context. Proc. R. Soc. B. 282, 20150299
Pokorny, T., Loose, D., Dyker, G., Quezada-Euán, J.G., Eltz, T. (2015) Dispersal ability of male orchid bees and direct evidence for long-range flights. Apidologie 46, 224–237
Potts, S.G., Biesmeijer, J.C., Kremen, C., Neumann, P., Schweiger, O., Kunin, W.E. (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353
Potts, S.G., Imperatriz-Fonseca, V., Ngo, H.T., Aizen, M.A., Biesmeijer, J.C., Breeze, T.D., Dicks, L.V., Garibaldi, L. A., Hill, R., Settele, J., Vanbergen, A.J. (2016). Safeguarding pollinators and their values to human wellbeing. Nature. 540, 220–229
Powell, A.H., Powell, G.V.N. (1987) Population dynamics of male Euglossine bees in Amazonian forest fragments. Biotropica. 19, 176–179
QGIS Development Team (2017) QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
R Core Team (2019) R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing
Ramírez, S.R., Eltz, T., Fujiwara, M.K., Gerlach, G., Goldman-Huertas, B., Tsutsui, N.D., Pierce, N.E. (2011) Asynchronous diversification in a specialized plant-pollinator mutualism. Science 333(6050), 1742–6. https://doi.org/10.1126/science.1209175
Rebelo, J.M.M., Moure, J.S. (1995) As espécies de Euglossa Latreille do nordeste de São Paulo (Apidae, Euglossinae). Rev. Bras. Zootec. 12(3), 445–446
Ribeiro, J.F., Walter B.M.T. (1998) Fitofisionomias do bioma Cerrado. In Cerrado : ambiente e flora. pp. 87–166, Planaltina. Available at: https://www.embrapa.br/buscade-publicacoes/-/publicacao/554094/fitofisionomias-do-bioma-cerrado
Rosa, J.F., Ramalho, M.I., Monteiro, D., Silva, M.D. (2015) Permeability of matrices of agricultural crops to Euglossina bees (Hymenoptera, Apidae) in the Atlantic Rain Forest. Apidologie 46, 691–702
Roubik, D. (Ed.) (1989) Ecology and Natural History of Tropical Bees (Cambridge Tropical Biology Series). Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511574641
Roubik, D.W. (2004) Sibiling species of Glossura and Glossuropoda in the Amazon region (Hymenoptera: Apidae: Euglossini). J Kansas Entomol. Soc. 77(3), 265–253
Sano, E.E., Rosa, R., Brito, J.L.S., Ferreira, L.G. (2010) Land cover mapping of the tropical savanna region in Brazil. Environ. Monit. Assess. 166, 113–124. https://doi.org/10.1007/s10661-009-0988-4
Santos, J.S., Dodonov, P., Emi, J., Oshima, F., Martello, F., Santos-Jesus, A., Ferreira, M.E., Silva-Neto, C.M., Ribeiro, M.C., Collevatti, R.G. (2021) Landscape ecology in the Anthropocene: an overview for integrating agroecosystems and biodiversity conservation. Perspect. Ecol.Conserv. 19(1), 21–32. https://doi.org/10.1016/j.pecon.2020.11.002
Santos, J.S., Silva-Neto, C.M., Sivla, T.C., Siqueira, K.N., Ribeiro, M.C., Collevatti, R.G. (2022). Landscape structure and local variables affect plant community diversity and structure in a Brazilian agricultural landscape. Biotropica, 54, 239-250. https://doi.org/10.1111/btp.13054
Silva, D.P., Aguiar, A.J.C., Melo, G.A.R., Anjos-Silva, E.J., De Marco, P. Jr. (2013) Amazonian species within the Cerrado savanna: new records and potential distribution for Aglae caerulea (Apidae: Euglossini). Apidologie 44, 673–683
Silva, D.P., De Marco, P. Jr. (2014) No evidence of habitat loss affecting the orchid bees Eulaema nigrita Lepeletier and Eufriesea auriceps Friese (Apidae: Euglossini) in the Brazilian Cerrado Savanna. Neotrop. Entomol. 43, 509–518
Silva, D.P., Nogueira, D.S., De Marco, P. Jr. (2017) Contrasting Patterns in Solitary and Eusocial Bees While Responding to Landscape Features in the Brazilian Cerrado: a Multiscaled Perspective. Neotrop. Entomol. 46, 264-274
Silva, J.M.C., Bates, J.M. (2002) Biogeographic Patterns and Conservation in the South American Cerrado: A Tropical Savanna Hotspot. BioScience 52, 225–234
Silveira, G.C., Freitas, R.F., Tosta, T.H.A., Rabelo, L.S., Gaglianone, M.C., Augusto, S.C. (2015) The orchid bee fauna in the Brazilian savanna: do forest formations contribute to higher species diversity? Apidologie 46, 197–208
Steffan-Dewenter, I., Munzenberg, U., Burger, C., Thies, C., Tscharntke, T. (2002) Scale-dependent effects of landscape Context on three pollinator guilds. Ecology 83, 1421–1432
Storck-Tonon, D., Morato, E.F., Melo, A.W.F., Oliveira, M.L. (2013) Orchid bees of forest fragments in Southwestern Amazonia. Biota Neotrop. 13, 133–141
Storck-Tonon, D., Peres, C.A. (2017) Forest patch isolation drives local extinctions of Amazonian orchid bees in a 26 years old archipelago. Biol. Conserv. 214, 270–277
Strassburg, B., Brooks, T., Feltran-Barbieri, R., Iribarrem, A., Crouzeilles, R., Loyola, R., Latawiec, A.E., Filho, F.J.B.O., Scaramuzza, C.A.M., Scarano, F.R., Soares-Filho, B., Balmford, A. (2017) Moment of truth for the Cerrado hotspot. Nat. Ecol. Evol. 1, 0099. https://doi.org/10.1038/s41559-017-0099
Tonhasca, A., Blackmer, J.L., Albuquerque, G.S. (2002) Abundance and diversity of Euglossine Bees in the fragmented landscape of the Brazilian Atlantic Forest. Biotropica. 34, 416-422
Tonhasca, A. Jr., Albuquerque, G.S., Blackmer, J.L. (2003) Dispersal of euglossine bees between fragments of the Brazilian Atlantic Forest. J. Trop. Ecol. 19, 99–102. https://doi.org/10.1017/S0266467403003122
Velazco, S.J.E., Villalobos, F., Galvão, F., De Marco, P. Jr. (2019) A dark scenario for Cerrado plant species: Effects of future climate, land use and protected areas ineffectiveness. Divers. Distrib. 25, 660–673. https://doi.org/10.1111/ddi.12886
Viana, B.F., Boscolo, D., Neto, E.M., Lopes, L.E., Lopes, A.V., Ferreira, P.A., Pigozzo, C.M., Primo, L.M. (2012) How well do we understand landscape effects on pollinators and pollination services? J. Pollinat. Ecol. 7, 31–41
Vilhena, O., Rocha, L.J., Garófalo, C.A. (2017) Male orchid bees (Hymenoptera: Apidae: Euglossini) in canopy and understory of Amazon várzea floodplain forest: I. microclimatic, seasonal and faunal aspects. Sociobiology. 64, 191–201
Wiens, J. (1989) Spatial Scaling in Ecology. Funct. Ecol. 3(4), 385–397. https://doi.org/10.2307/2389612
Wikelski, M., Moxley, J., Eaton-Mordas, A., López-Uribe, M.M., Holland, R., Moskowitz, D., Roubik, D.W., Kays, R. (2010) Large-range movements of Neotropical orchid bees observed via radio telemetry. PLoS ONE 5, E10738
Williams, N.M., Crone, E.E., Roulston, T.H., Minckley, R.L., Packer, L., Potts, S.G. (2010) Ecological and life-history traits predict bee species responses to environmental disturbances. Biol. Conserv. 143, 2280–2291
Winfree, R., Aguilar, R., Vázquez, D.P., LeBuhn, G., Aizen, M.A. (2009) A meta‐analysis of bees' responses to anthropogenic disturbance. Ecology 90, 2068–2076. https://doi.org/10.1890/08-1245.1
Winfree, R., Bartomeus, I.,Cariveau, D.P. (2011) Native Pollinators in Anthropogenic Habitats. Annu. Rev. Ecol. Evol. Syst. 42:1–22. https://doi.org/10.1146/annurev-ecolsys-102710-145042
Zuur, A.F., Ieno, E.N., Walker, N.J., Saveliev, A.A., Smith, G.M. (2009) Mixed Effects Models and Extensions In Ecology with R. Springer Science + Business Media, New York
Acknowledgements
We thank Arthur Ângelo Bispo, Paulo De Marco Jr, and Danilo Boscolo for discussions and valuable suggestions.
Funding
This study was supported by grants to the research network PELD COFA supported by CNPq/MCT/CAPES/FAPEG (project no. 441278/2016–7), FAPEG (project no. 201710267000331 and 202010267000404), and CAPES/PROCAD (project no. 88881.068425/2014–01). FGS received a fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and JSS received a CAPES postdoctoral fellowship and a postdoctoral grant from São Paulo Research Foundation (FAPESP, process n° 2019/09713-6). FM received a postdoctoral fellowship from the Foundation of Research Support and Development (FADESP). MCR thanks FAPESP (processes nº 2013/50421-2, 2020/01779-5, 2021/08534-0, and 2021/10195-0) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - processes nº 442147/2020-1; 402765/2021-4, and 313016/2021-6) for their financial support. RGC, MCR, and DPS (process nº 304494/2019-4) are continuously supported by productivity grants from CNPq, which we gratefully acknowledge.
Author information
Authors and Affiliations
Contributions
FGS and DPS conceived designed experiments; all authors participated in the interpretation of the data; FGS, JSS, and FM performed the analysis; all authors wrote the paper.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Sara Diana Leonhardt
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Sousa, F.G., dos Santos, J.S., Martello, F. et al. Natural habitat cover and fragmentation per se influence orchid-bee species richness in agricultural landscapes in the Brazilian Cerrado. Apidologie 53, 20 (2022). https://doi.org/10.1007/s13592-022-00925-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-022-00925-6