Abstract
Bumble bees (Bombus spp.) have been commercially propagated for over three decades. As the environmental conditions experienced by commercial bumble bees differ greatly from those experienced by wild bumble bees, commercial rearing of bumble bees may cause phenotypic changes. Here, we compare the foraging behavior and size of worker bumble bees (Bombus impatiens) from commercial and wild colonies. For this experiment, we measured worker body size, recorded if the workers returned with pollen, and examined the contents of pollen loads via microscopy. We found that, while commercial and wild bumble bees foraged on similar communities of flowers, wild bumble bees returned to colonies with purer pollen baskets (higher proportion of the most common species) and were more likely to return to the colony with pollen than their commercial counterparts. Commercial bumble bees were also smaller than wild bees. Our work highlights differences between commercial and wild bumble bees, in addition to raising important unanswered questions about the mechanism and drivers of these differences.
Similar content being viewed by others
1 Introduction
The use of commercially propagated animals as study subjects is common in biological research. Though these animals are often assumed to represent their wild counterparts, the environmental conditions and selective pressures experienced by commercially propagated animals may differ from those experienced by animals in the wild. Differentiation between domesticated and wild organisms has been well characterized across a wide range of taxa, including plants (Harlan et al. 1973), mammals (Trut et al. 2009), and fish (Huntingford 2004), though relatively few studies have addressed the impacts of domestication and commercial rearing on insects (Lecocq 2019). Nevertheless, many species of commercially propagated insects play important roles as scientific subjects: for example, the availability of commercially propagated mason bees (Osmia spp.), honey bees (Apis mellifera), and several species of bumble bees (e.g., Bombus terrestris and B. impatiens) has facilitated studies of both social and solitary bees (Pitts-singer and Cane 2011).
The common eastern bumble bee (B. impatiens), a social pollinator native to the Eastern United States, has been propagated commercially since 1990 (Velthuis and van Doorn 2006). Though bumble bees have a relatively short history of captive rearing (Velthuis and van Doorn 2006), phenotypic changes in domestic or managed populations are often induced in as few as five generations of captive breeding for other taxa (e.g., hatchery fish) (Araki et al. 2008). Commercial bumble bee populations might experience limited gene flow from wild populations, as the producers of commercial bumble bees maintain their own source populations (Velthuis and van Doorn 2006). Artificial conditions are likely to be stressful for bumble bees, as rates of colony success can vary greatly according to rearing techniques (Gurel and Gosterit 2008; Imran et al. 2016) and more than half of wild-caught queens may fail to establish colonies in laboratory conditions (Strange 2010). This stress may lead to selection for performance in lab conditions: previous research has shown commercial B. terrestris to produce significantly more gynes and workers than a native genotype in a common garden laboratory rearing experiment, with hybridization between commercial and native genotypes generally resulting in intermediate expression of these colony traits (Gosterit and Baskar 2016). This difference may be unsurprising, given producers of commercial bumble bees are known to select for colonies that establish quickly and grow rapidly (Yoon et al. 2011).
Phenotypic differentiation between commercial and wild organisms could also originate solely from differences in rearing environment. For example, many research animals are fed ad lib, which is suspected to lead to the expression of gyne-like characteristics in workers, at least for the social insect Polistes (Jandt et al. 2015). Bumble bees reared in artificial conditions may have no experience handling flowers and are typically fed Apis-collected pollen (Velthuis and van Doorn 2006), which is lower in protein than pollen collected by bumble bees (Leonhardt and Blüthgen 2012). In addition, producers of commercial bumble bees can manipulate the phenology of colonies by altering the hibernation period of queens (Velthuis and van Doorn 2006). This flexibility allows commercial greenhouse growers to deploy large bumble bee colonies in the early spring, when wild bumble bee workers are not yet abundant. During field studies, it is not unheard of for researchers to put out large colonies (approximately 100 workers strong) as early as May, when B. impatiens queens are typically still looking for nest sites (Drummond 2016; Gervais et al. 2020), or to put out similarly sized colonies at different times of the year during a single experiment (e.g., Osborne et al. 1999). Furthermore, although natural B. impatiens and B. terrestris nest sites are typically found belowground (Plath et al. 1922), commercial colony nest boxes are often installed above the ground to prevent nests from flooding (Osborne et al. 1999; Westphal et al. 2006) and are likely less well insulated from daytime temperatures. These, and other common practices associated with the use of commercial bumble bees in research, have unknown impacts on the behavior of workers once colonies are placed outdoors.
In this paper, we investigate whether inference from commercially propagated colonies of B. impatiens differs from inference from wild colonies. Specifically, we present a field experiment designed to compare size and foraging behavior of wild B. impatiens workers and workers from commercial colonies installed in the field. As metrics of behavior, we monitored the proportion of workers returning to the nest with pollen and identified the contents of pollen loads to morphospecies. Identifying pollen grains to morphospecies allowed us to assess the purity of each pollen load (i.e., the proportion of pollen grains representing the most common morphospecies in the pollen load). We also measured the intertegular span (IT span) of captured workers as they returned to the nest. Behavioral or morphological differences between bees from wild and commercial colonies could reflect a range of causes, including differences in colony phenology, age, and demographic history (e.g., methods of establishing colonies in the lab), as well as possible behavioral and genetic differences due to propagation methods per se. In our experiment, including these differences was necessary because we want to represent commercial colonies as they are typically used, and at least some aspects of propagation are proprietary (trade secrets). Thus, if we conclude that bees from commercial colonies are similar to bees from wild colonies, then we can conclude generally that commercially propagated bumble bees are good surrogates for their wild counterparts (at least for the traits we measured). If we find differences, our work could provide motivation for further research into the mechanisms behind these differences.
2 Methods
2.1 Study site
Appleton Farms (~1000 acres) is located in Ipswich, Massachusetts, and is maintained by the Trustees of Reservations. We selected a 0.3-ha meadow as our study site because of its relatively high worker bee density (Pugesek, personal observation). This meadow is mowed annually in the late fall to prevent succession.
2.2 Pollen collection
We collected pollen from 8 commercial and 8 wild B. impatiens colonies over the course of the study period. During each year of the study (2017 and 2018), four commercial colonies (Natupol Excel Observation, Koppert Biological Systems) were installed at the edges of the study site (see Appendix 1). Colonies were installed in July; each colony was placed on a wooden pallet and shaded with a tarp tent, in accordance with the recommendations of the supplier to provide shade on all sides of the colony. Colonies were fed sugar water (“Bee Happy,” Koppert Biological Systems) for a period of 1 week, after which we removed the nectar reservoir (included with the artificial nest box) to minimize the effects of feeding (Martin et al. 2018). Colonies were then left to acclimate for an additional 2 weeks, an acclimation period similar or greater than that of other studies that use commercial bees as study subjects (Osborne et al. 2008; Couvillon et al. 2010). By the time we began collecting data, we expected high turnover of foraging workers, as their life span in the wild is short (< 20 days in a field study of an ecologically similar species, B. vosnesenskii (Kerr et al. 2019). During this 3-week acclimation period, we located four wild B. impatiens colonies during each year of the study by freely searching the study site for workers entering or exiting colony entrances (Iles et al. 2019). Although commercial colonies were located at the edge of our study meadow (see Appendix 1), the field we worked in was quite small, less than half a hectare.
After the acclimation period was complete, workers were netted as they returned to their nest site for about 1 week. To induce cold anesthesia, workers were placed in vials on ice for 5–10 min. For each captured individual, we checked for the presence or absence of pollen loads and measured the intertegular (IT) span, the shortest distance between the two-wing tegulae, a common measure of bumble bee body size (Greenleaf et al. 2007). In 2017, we also collected pollen loads from workers nondestructively using a sterilized razor blade and preserved pollen loads on ice.
After measurements were complete, workers were marked using a Sharpie paint pen and released. Marked workers were not repeatedly measured. All workers were collected between 8 am and 3 pm. We alternated collecting workers from wild and domestic colonies (collecting 1 worker from each colony before rotating to the next) to better ensure that time of day did not bias sampling (Free 1955; Peat et al. 2005).
2.3 Pollen identification
Pollen loads were suspended in 100-μl ethanol, and 20 μl of this solution was combined with 40-μl fuchsin gel (Beattie 1971) and plated onto microscope slides. A ZEISS Axio Scope A1 microscope was used to view pollen grains, and pollen grains were imaged and measured using Spot 5.2 software. Pollen grains were categorized to one of 13 morphospecies, differentiated by size, surface markings, and shape, though they were not assigned to specific plant species or genera (Appendix 2). For each slide, 200 pollen grains were identified to morphospecies, with 40 pollen grains were sampled haphazardly from each corner and an additional 40 sampled from the center of the slide.
2.4 Statistical methods
All statistical analyses were performed in R, version 4.0.2 (RStudio Team 2019). We compared the body size of workers originating from commercial and wild bees using a linear mixed effects model (lme4), derived using restricted maximum likelihood (REML = TRUE). Bee origin (commercial or wild) and year (2017 or 2018) were included as fixed effects, and colony ID was included as a random effect (Bates et al. 2015). (Note that comparison of body size could only be done with foraging workers because B. impatiens colonies are subterranean and cannot be nondestructively monitored.) Similarity, pollen load presence or absence was analyzed using binomial family, generalized linear mixed effects models (lme4) (Bates et al. 2015) with bee origin and year as fixed effects and colony ID as a random effect. The significance of each fixed term was determined using type II marginal hypothesis tests implemented with the ANOVA function in the R package car (Fox and Weisberg 2019).
To evaluate the purity of pollen loads, we determined the most common pollen morphospecies contained in each pollen load and calculated the proportion of pollen grains representing this morphospecies from the total sample. We used linear models to analyze data with bee origin as a fixed effect. To meet assumptions of normality, data were logit transformed after rescaling from 0.025 to 0.0975. The significance of bee origin was determined using type II marginal hypothesis tests implemented with the ANOVA function.
We compared the morphospecies composition of pollen loads collected by commercial and wild bumble bees using a permutational multivariate analysis of variance (PERMANOVA) with bumble bee origin as a predictor variable, implemented with the function adonis2 (package vegan) (Anderson 2017) in R, using Bray–Curtis dissimilarity indices. Data were square root transformed to minimize the effects of most abundant groups. We performed an additional, unconstrained analysis (non-metric multidimensional scaling ordination, i.e., NMDS) to visualize pollen morphospecies abundance data. NMDS was performed using a two-dimensional solution (stress = 0.1015) and Bray–Curtis dissimilarity indices (function metaMDS, package vegan). Data were square root transformed prior to analysis (once again, to minimize the effects of most abundant groups). The plot produced from NMDS is not meant to represent the results of our constrained analyses (PERMANOVA), as approaches for dimensionality reduction differ between constrained and unconstrained analyses (Paliy and Shankar 2016; Scott and Crone 2021). Our NMDS analysis simply serves to visualize data in a reduced dimensional space.
3 Results
Over the course of 2 years, a total of 271 workers were sampled from 16 colonies (4 colonies/treatment/year): in 2017, we collected 102 commerical workers and 101 wild workers, while in 2018, we collected 29 commercial workers and 39 wild workers.
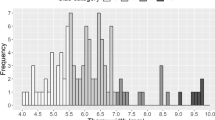
Commercial worker bumble bees were smaller than their wild counterparts (χ2= 10.618, df = 1, P = 0.001) (Figure 1). In 2017, average IT span was 3.30 (SE = 0.06) mm for commercial and 3.43 (SE = 0.06) mm for wild workers, while in 2018, average IT span was 3.45 (SE = 0.10) mm for commercial and 3.92 (SE = 0.09) mm for wild workers. Worker bumble bees collected in 2017 were also significantly smaller than bees collected in 2018 (χ2= 18.705, df = 1, P < 0.001) (Figure 1). There was a significant interaction between these main effects (χ2= 4.679, df = 1, P = 0.031): the size difference between commercial and domestic bees was larger in 2018 (Figure 1). Worker body size did not differ much among colonies within groups, as indicated by random effect standard deviation of 0.06 for IT span.
Workers from wild colonies were more likely to return to the colony with pollen than workers from commercial colonies (χ2= 6.72, df = 1, P = 0.010) (Figure 2). In 2017, 34.4% (SE = 7.1) of commercial workers and 49.8% (SE = 7.7) of wild workers returned to the nest with pollen, while in 2018, 22.0% (SE = 9.0) of commercial workers and 58.9% (SE = 9.9) of wild workers returned with pollen. There was no significant effect of year on the proportion of workers found returning to the colony with pollen (χ2= 0.010, df = 1, P = 0.922), nor was there a significant interaction (χ2= 1.544, df = 1, P = 0.214). Pollen foraging differed moderately among colonies within groups, as indicated by random effect standard deviation of 0.46 for the proportion carrying pollen (note that this SD is on a logit-scale)
According to PERMANOVA, the composition of flower morphospecies communities visited by commercial and wild bumble bees did not differ (F1, 70= 1.5691, R2= 0.02192, P = 0.193). In addition, NMDS analysis showed no clear separation of contents of pollen loads collected by commercial and wild bees (Figure 3). Both commercial and wild bumble bees tended to visit more than one flower morphospecies during foraging bouts: 71 of 72 of pollen loads contained pollen from more than one morphospecies. However, the pollen loads collected by commercial bumble bees were not as pure than those collected wild bees (F1, 70= 9.317, P = 0.003) (Figure 4). Together, these results suggest that while commercial and wild colonies collected the same diversity of pollen, individual workers were more specialized in wild colonies.
4 Discussion
Workers from commercial and wild bumble bee colonies differ in terms of foraging behavior. While commercial and wild workers visited similar communities of flowers (Figure 3), commercial workers were less likely to return to the colony with pollen. Our results also suggest wild bumble bees may be more specialized foragers relative to commercial bees, as wild bees collected purer pollen loads. Past studies have shown that domesticated insects may be as effective as their wild counterparts when handling food, at least in highly controlled laboratory environments (Cohen 2000; Hagler 2009). However, in other taxa, domestication often leads to changes in foraging behavior. For example, domestic pigs (Sus scrofa) and fowl (Gallus gallus domesticus) move less during foraging (Gustafsson et al. 1999; Andersson et al. 2001; Schütz et al. 2001), suggesting that domestic animals are not as willing to allocate resources to highly energetic foraging behaviors. In contrast, brown trout (Salmo trutta) reared in hatcheries tend to move more and feed less while foraging than wild brown trout (Bachman 1984). Domestication can clearly affect foraging behavior of many taxa, including B. impatiens.
Foraging workers from commercial colonies were smaller than those of wild origin (Figure 1). Our results contrast with those of Ings et al. (2006), who found that foragers from non-native, commercial B. terrestris colonies tended to be larger than foragers from colonies of native subspecies. One reason for this difference may be that Ings et al. (2006) compared a native subspecies of B. terrestris, B. t. audax, to a commercially available non-native subspecies, B. t. dalmatinus. Thus, differences in body size observed by Ings et al. (2006) may have been driven by differences between subspecies, as opposed to commercialization. In contrast, there are no recognized subspecies of B. impatiens. In other insect taxa, artificial selection may lead to larger body sizes (e.g., silkworms) (Lecocq 2019), though captive rearing has led to decreased body size for some species of insects (Cohen 2000; Schultz et al. 2009). In the case of bumble bees, it is possible that selection for larger colony sizes (i.e., colonies with more workers) has led to an inadvertent selective pressure for smaller workers. Among larger colonies of B. impatiens, workers tend to be smaller (Couvillon et al. 2010). Production of larger workers requires more resources, and thus there are trade-offs between producing larger workers and producing a larger number of workers for bumble bees (Kerr et al. 2019).
In our study, another cause of variation in body size could be the developmental age or stage of bees in domesticated vs. wild colonies (Chole et al. 2019). Colonies purchased from Koppert (and other commercial suppliers) are classified by the number of workers, rather than colony age or developmental history. Therefore, we did not know the demographic composition of these colonies. We were also unable to infer the age or developmental state of wild colonies, as we located established colonies in July, when colonies were large and more conspicuous. For other bumble bee species, anecdotal evidence has suggested bumble bee worker body size increases as the colony develops (Chole et al. 2019). However, to our knowledge, only one study (Couvillon et al. 2010) has addressed ontogeny of worker body size distribution in B. impatiens colonies, finding no evidence average worker body size changes through time. It is worth noting that the Couvillon et al. study took place entirely in the laboratory — it is possible that other patterns could arise in the field. Understanding the mechanisms of body size differences would require some combination of field studies done in close collaboration with commercial suppliers and common garden studies with colonies founded by mated queens either from the wild or from a commercial producer (e.g., Gosterit and Baskar 2016). Nonetheless, colonies of unknown demographic history are now routinely used in ecological research to represent wild bumble bees (Drummond 2016; Gervais et al. 2020). Our study suggests that further research into the differences between commercial and wild colonies would help us understand how to interpret these studies.
Similarly, many factors could contribute to the differences we measured in foraging behavior of workers from commercial and wild colonies. Since our goal was to compare inference from commercial colonies as they are typically used, we installed them as directed by the manufacturer, rather than in a way that was comparable to the conditions experienced by wild bumble bees. Bombus impatiens colonies usually occur underground (Pugesek and Crone in press); in 2017, we excavated two of these nests at our field site, and they were located at the end of several feet of underground tunnels (presumably in abandoned small mammal burrows). These conditions are not feasible to recreate for colonies in nest boxes; instead, Koppert and other commercial suppliers recommend placing colonies aboveground beneath trees or under tents to prevent colonies from overheating. The use of aboveground boxes is a common technique to study bumble bees in the field, even with colonies propagated from wild-caught queens (Kerr et al. 2019; Malfi et al. 2019). If these differences in colony position affect foraging behavior of bees in our study, they would also lead to similar confounding factors in many studies that use bees in aboveground boxes to represent wild bees.
Although commercial colonies were installed under trees toward the edges of the field site, we suspect that colony location was not a significant factor in the differences we observed because all colonies were located in a small (< 1 ha) field (Appendix 1). Wild colonies were often within 50 m of commercial colonies. To our knowledge, there are no published estimates of B. impatiens foraging ranges; however, other species of bumble bees readily forage more than 250 m from nest sites (Osborne et al. 1999; Walther-Hellwig and Frankl 2000), with some workers foraging at distances greater than a kilometer (Osborne et al. 2008; Walther-Hellwig and Frankl 2000). If B. impatiens foraging ranges are similar, we would expect that foraging ranges of commercial and wild bumble bees would overlap almost completely (Appendix1). In addition, we did not observe any differences in the overall pollen composition collected by workers from commercial vs. wild colonies (Figure 3), suggesting that they were foraging for similar resources. Rather, the difference was in the purity of loads brought back by single workers.
It is tempting to speculate that the two effects we observed — differences in body size and foraging behavior — are related. In some bumble bee species, larger workers collect nectar at a faster rate than smaller bumble bees (Goulson et al. 2002). In fact, Ings et al. (2006) cites differences in foraging worker body size as a potential driver of differences in nectar return between lab-reared B. t. audax and commercial B. t. dalmatinus. In that study, commercial bumble bees weighed 20–70% more than wild workers (depending on the study site location), compared to a 9% difference of average IT span (3.35 vs. 3.63 mm) in our study. Kerr et al. (2019) observed essentially no change in the probability that larger B. vosnesenskii workers would carry pollen over this size range, although very large workers (4.5 mm IT span) were more likely to forage for pollen, and carried slightly more pollen per trip. In our data, IT span was not a significant predictor of the probability workers would carry pollen, after accounting for colony origin (Appendix 3). Based on these results, it may be parsimonious to conclude that differences in body size and pollen foraging result from different causes.
Large workers have a better memory and are faster learners than small workers (Worden et al. 2005; Riveros and Gronenberg 2009). Thus, larger workers may be more suited to challenging or complex foraging tasks like collecting pollen or handling flowers. This result could explain the difference in pollen purity, if we reason that “majoring” on a species during foraging bouts is a complex behavior. However, it could be argued that “majoring” simplifies the foraging process and would require less cognitive capability. Like many aspects of the effects of domestication on bumble bees, the causes and consequences of this difference would be a fascinating direction for future research.
The use of commercial bumble bees, both for pollination services and for research, has increased tremendously since the late 1980s (Velthuis and van Doorn 2006), and there is growing interest in identifying new candidate species for commercialization (Baur et al. 2019). Commercially propagated bumble bees (B. impatiens and/or B. terrestris) have been used to evaluate a wide range of foraging behaviors: floral preference (Vaudo et al. 2014), worker bumble bee foraging ranges or trip duration (Osborne et al. 1999, 2008; Westphal et al. 2006), and worker foraging performance or resource return (Goulson et al. 2002; Raine and Chittka 2008; Feltham et al. 2014). Researchers have frequently used commercial colonies to evaluate foraging worker behavioral response to colony manipulation of pollen or nectar stores (Plowright et al. 1999; Kitaoka and Nieh 2009; Hendriksma et al. 2019) or with exposure to insecticides (Feltham et al. 2014). If our results represent commercially propagated bumble bees in general, they inform our inference about which kinds of studies commercial bumble bees would best represent wild bumble bee colonies. For example, inference about pollen foraging (Leonhardt and Blüthgen 2012) and floral preferences of workers (Vaudo et al. 2014; Drummond 2016) may be transferrable from commercial to wild populations, given that we observed commercial and wild bumble bees to visit similar communities of flowers (at least in terms of morphospecies). However, inference about net resource return (Goulson et al. 2002; Feltham et al. 2014) or preferences for pollen over nectar (Plowright et al. 1999; Peat and Goulson 2005; Hendriksma et al. 2019) may be less transferrable, if (as suggested by our results) wild bumble bees generally return with pollen more frequently or are more likely to major on particular species while foraging for pollen. This study highlights that, although commercial bumble bees are similar to wild bumble bees in many ways, they are not perfectly substitutable.
In spite of these differences, we do not seek to completely discourage the use of commercial bumble bee colonies in research. Many of the published studies which use commercial bumble bees as study subjects would not otherwise have been possible, and there are many benefits to using similarly sized bumble bee colonies in experiments. However, we believe it is important to recognize the caveat that the bees used in these experiments may well differ from their wild counterparts. Although challenging, it is possible to locate bumble bee colonies in the field with large enough sample sizes to ask focused ecological questions (cf. Harder 1986; Pugesek and Crone 2021). In the future, it will be useful to complement research on domesticated bumble bees with research on wild colonies.
References
Anderson MJ (2017) Permutational multivariate analysis of variance (PERMANOVA). In: Wiley StatsRef: Statistics Reference Online. John Wiley & Sons, Ltd, Chichester, UK, pp 1–15
Andersson M, Nordin E, Jensen P (2001) Domestication effects on foraging strategies in fowl. Appl Anim Behav Sci 72:51–62. https://doi.org/10.1016/S0168-1591(00)00195-7
Araki H, Berejikian BA, Ford MJ, Blouin MS (2008) Fitness of hatchery-reared salmonids in the wild. Evol Appl 1:342–355. https://doi.org/10.1111/j.1752-4571.2008.00026.x
Bachman RA (1984) Foraging behavior of free-ranging wild and hatchery brown trout in a stream. Trans Am Fish Soc 113:1–32.
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1-48. https://doi.org/10.18637/jss.v067.i01
Baur A, Strange JP, Koch JB (2019) Foraging economics of the Hunt bumble bee, a viable pollinator for commercial agriculture. Environ Entomol 48:799–806. https://doi.org/10.1093/ee/nvz075
Beattie AJ (1971) A technique for the study of insect-borne pollen. Pan-Pac Entomol 47:82
Chole H, Woodard SH, Bloch G (2019) Body size variation in bees: regulation, mechanisms, and relationship to social organization. Curr Opin Insect Sci 35:77–87. https://doi.org/10.1016/j.cois.2019.07.006
Cohen, AC (2000) Feeding fitness and quality of domesticated and feral predators: Effects of long-term rearing on artificial diet. Biological Control 17:50–54
Couvillon MJ, Jandt JM, Duong N, Dornhaus A (2010) Ontogeny of worker body size distribution in bumble bee (Bombus impatiens) colonies. Ecol Entomol 35:424–435. https://doi.org/10.1111/j.1365-2311.2010.01198.x
Drummond FA (2016) Behavior of bees associated with the wild blueberry agro-ecosystem in the USA. Int J Entomol Nematol 2:27–41
Feltham H, Park K, Goulson D (2014) Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology 23:317–323. https://doi.org/10.1007/s10646-014-1189-7
Fox J, Weisberg, S (2019) An R companion to applied regression, Third Edition, Sage Publications, Thousand Oaks
Free JB (1955) The collection of food by bumblebees. Insectes Soc 2:303–311
Gervais A, Fournier V, Bélisle, M (2020) Agricultural landscape composition affects the development and life expectancy of colonies of Bombus impatiens. Ecosphere 11: e03142. https://doi.org/10.1002/ecs2.3142
Gosterit A, Baskar VC (2016) Impacts of commercialization on the developmental characteristics of native Bombus terrestris (L.) colonies. Insectes Soc 63:609–614. https://doi.org/10.1007/s00040-016-0507-x
Goulson D, Peat J, Stout JC, et al (2002) Can alloethism in workers of the bumblebee, Bombus terrestris, be explained in terms of foraging efficiency? Anim Behav 64:123–130. https://doi.org/10.1006/anbe.2002.3041
Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153:589–596. https://doi.org/10.1007/s00442-007-0752-9
Gurel F, Gosterit A (2008) Effects of different stimulation methods on colony initiation and development of Bombus terrestris L. (Hymenoptera: Apidae) queens. Appl Entomol Zool 43:113–117. https://doi.org/10.1303/aez.2008.113
Gustafsson M, Jensen P, de Jonge FH, Schuurman T (1999) Domestication effects on foraging strategies in pigs (Sus scrofa). Appl Anim Behav Sci 62:305–317. https://doi.org/10.1016/S0168-1591(98)00236-6
Hagler J (2009) Comparative studies of predation among feral, commercially-purchased, and laboratory-reared predators. BioControl 54:351–361. https://doi.org/10.1007/s10526-008-9173-x
Harder L (1986) Influences on the density and dispersion of bumble bee nests (Hymenoptera: Apidae). Ecography 9: 99–103 http://doi.wiley.com/10.1111/j.1600-0587.1986.tb01196.x
Harlan JR, de Wet JMJ, Price EG (1973) Comparative evolution of cereals. Evolution 27:311–325 https://doi.org/10.1111/j.1558-5646.1973.tb00676.x
Hendriksma HP, Toth AL, Shafir S (2019) Individual and colony level foraging decisions of bumble bees and honey bees in relation to balancing of nutrient needs. Front Ecol Evol 7:1–12. https://doi.org/10.3389/fevo.2019.00177
Huntingford FA (2004) Implications of domestication and rearing conditions for the behavior of cultivated fishes. Fish Biology 65:122-142. https://doi.org/10.1111/j.0022-1112.2004.00562.x
Iles DT, Pugesek G, Kerr NZ, et al (2019) Accounting for imperfect detection in species with sessile life cycle stages: a case study of bumble bee nests. J Insect Conserv 23:945–955. https://doi.org/10.1007/s10841-019-00179-1
Imran M, Ahmad M, Naeem M, et al (2016) Effect of different types of boxes on rearing of bumble bee, Bombus terrestris. Pak J Zool 49:169–174. https://doi.org/10.17582/journal.pjz/2017.49.1.169.174
Ings TC, Ward NL, Chittka L (2006) Can commercially imported bumble bees out-compete their native conspecifics? J Appl Ecol 43:940–948. https://doi.org/10.1111/j.1365-2664.2006.01199.x
Jandt JM, Thomson JL, Geffre AC, Toth AL (2015) Lab rearing environment perturbs social traits: a case study with wasps. Behavioral Ecology 26 (5):1274-1284
Kerr NZ, Crone EE, Williams NM (2019) Integrating vital rates explains optimal worker size for resource return by bumblebee workers. Funct Ecol 33:467–478. https://doi.org/10.1111/1365-2435.13251
Kitaoka TK, Nieh JC (2009) Bumble bee pollen foraging regulation: role of pollen quality, storage levels, and odor. Behav Ecol Sociobiol 63:625–625. https://doi.org/10.1007/s00265-008-0707-0
Lecocq T (2019) Insects: The Disregarded Domestication Histories. In: F. Teletchea (ed) Animal Domestication. IntechOpen, London, pp 1-33
Leonhardt SD, Blüthgen N (2012) The same, but different: pollen foraging in honeybee and bumblebee colonies. Apidologie 43:449–464. https://doi.org/10.1007/s13592-011-0112-y
Malfi RL, Crone E, Williams N (2019) Demographic benefits of early season resources for bumble bee (B. vosnesenskii) colonies. Oecologia 191 (2):377-388
Martin CD, Toner C, Fountain MT, Brown MHF (2018) Busier bees: increasing nest traffic in commercial bumblebee colonies. J Pollinat Ecol 25:7–15. https://doi.org/10.26786/1920-7603(2018)21
Osborne JL, Clark SJ, Morris RJ, et al (1999) A landscape-scale study of bumble bee foraging range and constancy, using harmonic radar. J Appl Ecol 36:519–533. https://doi.org/10.1046/j.1365-2664.1999.00428.x
Osborne JL, Martin AP, Carreck NL, et al (2008) Bumblebee flight distances in relation to the forage landscape. J Anim Ecol 77:406–415. https://doi.org/10.1111/j.1365-2656.2007.01333.x
Paliy O, Shankar V (2016) Application of multivariate statistical techniques in microbial ecology. Mol Ecol 25: 1032–1057. https://doi.org/10.1111/mec.13536
Peat J, Goulson D (2005) Effects of experience and weather on foraging rate and pollen versus nectar collection in the bumblebee, Bombus terrestris. Behav Ecol Sociobiol 58:152–156. https://doi.org/10.1007/s00265-005-0916-8
Peat J, Tucker J, Goulson D (2005) Does intraspecific size variation in bumblebees allow colonies to efficiently exploit different flowers? Ecol Entomol 30:176–181. https://doi.org/10.1111/j.0307-6946.2005.00676.x
Pitts-singer TL, Cane JH (2011) The alfalfa leafcutting bee, Megachile rotundata: The world’s most intensively, managed solitary bee∗. Annu Rev Entomol 56:221–237. https://doi.org/10.1146/annurev-ento-120709-144836
Plath OE (1922) Notes on the Nesting Habits of Several North American Bumblebees. Psyche: A Journal of Entomology 29 (5-6):189-202
Plowright CMS, Cohen-Salmon D, Landry F, Simonds V (1999) Foraging for nectar and pollen on thistle flowers (Cirsium vulgare) and artificial flowers: How bumble bees (Bombus impatiens) respond to colony requirements. Behaviour 136:951–963. https://doi.org/10.1163/156853999501667
Pugesek G, Crone EE (in press) Contrasting effects of land cover on nesting habitat use and reproductive output for bumble bees. Ecosphere.
Raine NE, Chittka L (2008) The correlation of learning speed and natural foraging success in bumble-bees. Proc R Soc B Biol Sci 275:803–808. https://doi.org/10.1098/rspb.2007.1652
Riveros AJ, Gronenberg W (2009) Olfactory learning and memory in the bumblebee Bombus occidentalis. Naturwissenschaften 96:851–856. https://doi.org/10.1007/s00114-009-0532-y
RStudio Team (2019). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/
Schultz CB, Dzurisin JD, Russell C (2009) Captive rearing of Puget blue butterflies (Icaricia icarioides blackmorei) and implications for conservation. J Insect Conserv 13:309-21. https://doi.org/10.1007/s10841-008-9174-1
Schütz KE, Forkman B, Jensen P (2001) Domestication effects on foraging strategy, social behaviour and different fear responses: a comparison between the red junglefowl (Gallus gallus) and a modern layer strain. Appl Anim Behav Sci 74:1–14. https://doi.org/10.1016/S0168-1591(01)00156-3
Scott ER, Crone EE (2021) Using the right tool for the job: the difference between unsupervised and supervised analyses of multivariate ecological data. Oecologia. https://doi.org/10.1007/s00442-020-04848-w
Strange JP (2010) Nest initiation in three North American bumble bees (Bombus): gyne number and presence of honey bee workers influence establishment success and colony size. J Insect Sci.10:130. doi:https://doi.org/10.1673/031.010.13001
Trut L, Oskina I, Kharlamova A (2009) Animal evolution during domestication: the domesticated fox as a model. BioEssays 31:349–360. https://doi.org/10.1002/bies.200800070
Vaudo AD, Patch HM, Mortensen DA, et al (2014) Bumble bees exhibit daily behavioral patterns in pollen foraging. Arthropod Plant Interact 8:273–283. https://doi.org/10.1007/s11829-014-9312-5
Velthuis HHW, van Doorn A (2006) A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37:421–451. https://doi.org/10.1051/apido:2006019
Walther-Hellwig K, Frankl R, (2000) Foraging habitats and foraging distances of bumblebees, Bombus spp. (Hym., Apidae), in an agricultural landscape. Journal of Applied Entomology 124 (7-8):299-306
Westphal C, Steffan-Dewenter I, Tscharntke T (2006) Foraging trip duration of bumblebees in relation to landscape-wide resource availability. Ecol Entomol 31:389–394. https://doi.org/10.1111/j.1365-2311.2006.00801.x
Worden BD, Skemp AK, Papaj DR (2005) Learning in two contexts: the effects of interference and body size in bumblebees. J Exp Biol 208:2045–2053. doi:https://doi.org/10.1242/jeb.01582
Yoon H-J, Lee K-Y, Kim M-A, Park I-G (2011) Agricultural utilization and year-round rearing techniques of bumblebees in Korea. Int J Ind Entomol 22:29–37. https://doi.org/10.7852/ijie.2011.22.2.29
Acknowledgements
We thank Annika Greenleaf for her assistance with field work and the Trustees of Reservations for allowing us access to their properties. A special thanks to Sarah Richman, Jake Francis, and Anne S. Leonard for providing feedback on this manuscript prior to publication.
Availability of data and material/code availability
All data associated with these analyses will be archived in Dryad Digital Repository upon acceptance of this manuscript.
Funding
Funding for this project was provided by the Deans’ Natural and Social Sciences and fellowship at Tufts University and Tufts Summer Scholars program. This work was conducted while E.E.C. was supported by NSF DEB 13-54224.
Author information
Authors and Affiliations
Contributions
All authors participated in the conception of ideas and aspects of study design. G. Pugesek and E. E. Crone wrote this manuscript and ran statistical analyses. G. Pugesek and C. Burtt led field work and data collection.
Corresponding author
Ethics declarations
Ethics approval/consent to participate
No ethics approval or consent to participate was required for this research.
Consent for publication
All authors have agreed to the submission and publication in Apidologie. All authors have reviewed this manuscript prior to publication.
Conflicts of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Mathieu Lihoreau
Les effets de la propagation commerciale sur le butinage et la taille des bourdons ( Bombus impatiens ).
Bombus / bourdon / taille du corps / comportement de recherche de nourriture / propagation commerciale / domestication.
Der Effekt der kommerziellen Vermehrung auf das Sammelverhalten und die Körpergröße von Hummeln ( Bombus impatiens ).
Bombus / Hummeln / Körpergröße / Sammelverhalten / kommerzielle Vermehrung / Domestizierung.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data from: The effects of commercial propagation on bumble bee (Bombusimpatiens) foraging and worker body size. https://doi.org/10.6084/m9.figshare.14527251.v1
Rights and permissions
About this article
Cite this article
Pugesek, G., Burtt, C.N. & Crone, E.E. The effects of commercial propagation on bumble bee (Bombus impatiens) foraging and worker body size. Apidologie 52, 887–898 (2021). https://doi.org/10.1007/s13592-021-00867-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-021-00867-5