Abstract
The parasitic mite Varroa destructor is a major threat to the European honey bee Apis mellifera. Beekeepers apply the miticide thymol directly within the hives to kill this parasitic mite. Thymol is repellent to bees and causes them to ventilate the hive, yet its impact on bee hygienic behaviours that prevent the spread of diseases has never been studied. We measured the efficiency of colonies at removing dead adult bees, uncapping dead pupal cells and removing dead brood in two miticide-free Australian environments where the mite is absent. Thymol increased the uncapping and removal of dead brood by 24 to 36% after 48 h at both locations but had no effect on the removal of dead adult bees. The increased removal of brood could enhance the effect of thymol on V. destructor, especially if bees preferentially remove cells infected with the mite.
Similar content being viewed by others
1 Introduction
Amongst the many challenges faced by the European honey bee, the parasitic mite Varroa destructor is arguably the most damaging agent to date (Ratnieks and Carreck 2010; Guzmán-Novoa et al. 2010). Varroa destructor is an obligate ectoparasitic mite that was originally found on the Asian honey bee Apis cerana, but switched hosts to parasitise A. mellifera during the early 1960s and subsequently, colonised European populations in the mid-1970s (Jong et al. 1982; Goodwin and Van Eaton 2001; Rosenkranz et al. 2010). The mite has a phoretic stage where mature individuals attach themselves onto adult honey bees and a reproductive phase where female mites lay eggs in sealed brood cells of pupating honey bee larvae (Goodwin and Van Eaton 2001; Wallner and Fries 2003). V. destructor causes damage by sucking the haemolymph of its host, which weakens the bee and facilitates the transmission of multiple viruses and other diseases (Boecking and Spivak 1999; Le Conte et al. 2010; Rosenkranz et al. 2010).
Varroa destructor is thought to have a greater impact on the European honey bees than on its original host due to differences in hygienic responses and natural defences to the mite. On Apis cerana, consequences of V. destructor infections are reduced by different physiological and behavioural responses, including the effective detection and removal of phoretic mites and infected brood (Peng et al. 1987; Rath and Drescher 1990; Rath 1999). Similar hygienic responses have been observed in A. mellifera, but they are usually insufficient in protecting the colony against the mite (Rosenkranz et al. 2010). In both species, workers can detect, uncap and remove abnormal capped and uncapped brood cells and dead bees from the colony (Spivak 1996; Evans and Spivak 2010). Two independent behaviours are involved in the removal of the capped brood: the uncapping of diseased cells sealed with an operculum and the removal of abnormal brood from the hive (Lapidge et al. 2002). These behaviours are carried out by workers aged between 15 and 20 days old (Gramacho and Spivak 2003; Evans and Spivak 2010; Gerdts 2014). Honey bees also remove dead adults from within hives (Trumbo and Robinson 1997). This is performed by specialised undertaker bees, aged ≥ 23 days old, that constitute only 1 to 2% of the worker bee population within a hive (Visscher 1983; Robinson and Page 1988; Trumbo et al. 1997). Hygienic behaviours are genetically determined and strongly rely on hygienic bees and undertakers detecting specific olfactory cues from dead or diseased individuals (Spivak and Gilliam 1993; Masterman et al. 2000; Spivak and Reuter 2001a; Gramacho and Spivak 2003). Hygienic behaviours are essential in eusocial insects as they reduce the transmission and accumulation of pathogens within a colony (Trumbo and Robinson 1997; Evans and Spivak 2010).
To control the spread of V. destructor, beekeepers initially used acaricides such as bromopropylate, coumaphos, diazinon, amitraz, flumethrin and fluvalinate (Marchetti et al. 1984; Kanga et al. 2010; Škerl et al. 2010; Berry et al. 2013; Coffey and Breen 2013; Charpentier et al. 2014). However, over time, V. destructor has developed a resistance to many of these pesticides (Faucon et al. 1995; Milani 1995, 1999; Coffey 2007; Charpentier et al. 2014). Furthermore, these chemicals were shown to increase superscedure events (early replacement of the queen) (coumaphos and fluvalinate, Berry et al. 2013), heighten mortality in adults and brood (coumaphos on adults and fluvalinate on brood and adults, Berry et al. 2013), reduce body weight in queens (fluvalinate, Haarmann et al. 2002), decrease the amount of lipids, carbohydrates and proteins in the haemolymph of workers (amitraz and flumethrin, Loucif-Ayad et al. 2010). Furthermore, these treatments often accumulate in the wax, which may impact the development of bee larvae and mite resistance to miticides in subsequent seasons (Boi et al. 2016). For these reasons, alternative treatments such as essential oils and organic acids have become increasingly attractive to the beekeeping industry (Mondet et al. 2011; Charpentier et al. 2014) although in high concentrations they also affect larval survival and mass (thymol, Charpentier et al. 2014).
Thymol is an acaricide that has rapidly risen in popularity and is now one of the main methods of control for V. destructor (Mondet et al. 2011). It was also suggested that thymol helps control the spread of chalkbrood and other parasites (Grobov et al. 1981; Colin et al. 1989; Costa et al. 2010). The effects of thymol on mites and honey bees remains largely understudied but it is believed that thymol affects these organisms by interfering with GABA receptors and enhancing GABA responses (Price and Lummis 2014). Thymol can repel honey bees, interact with olfactory receptors and induce fanning behaviour in European honey bees (Mondet et al. 2011). This suggests that thymol could negatively affect hygienic behaviours in honey bees by interfering with the chemical cues that trigger the removal of diseased or dead brood and adults (Mondet et al. 2015). Several olfactory signals indicating larval health and/or decay are involved in the removal of dead adults and larvae (Wilson et al. 1958, Gordon 1983; Choe et al. 2009; McAfee et al. 2018), but no published research exists investigating the effects of thymol application on hygienic responses. It is imperative that we understand how thymol may impact the behaviours of honey bees as good hygienic behaviour is an important behavioural mechanism of a resistance against V. destructor and other pathogens (Goodwin and Van Eaton 2001; Evans and Spivak 2010).
Australia is currently the only continent remaining free of V. destructor, thus providing a rare and likely brief opportunity to conduct experiments associated with V. destructor treatments without the mite, and other miticides residues, which may confound experimental data (Iwasaki et al. 2015). It is also essential that we understand the effects of miticide treatments and determine mite control methods that have minimal impacts on bee behaviour in the event of V. destructor invading Australia (Hafi et al. 2012). Here, we set out to assess the impact of Apiguard®, a commercial formulation of thymol, on European honey bees to determine its impact on the uncapping and removal of dead brood and on the undertaking of dead bees. We replicated this experiment in two different Australian environments: in Hobart (Tasmania) and Richmond (New South Wales). Our results provide new insights on the impacts of thymol on European honey bee behaviours and will help to select and design adequate V. destructor treatments.
2 Materials and methods
2.1 Hive history and study site
In Hobart, 16 eight-frame, Langstroth hives (thymol n = 8, control n = 8) were housed at the University of Tasmania’s apiary at the Horticultural Science Research Centre, Sandy Bay (42° 54′ 29″ S; 147° 19′ 22″ E). Each hive was made of three to four supers (‘ideal’ boxes), two supers contain the same comb surface as a normal box. These hives were established by splitting and the queens were replaced in January 2016 using open-mated, commercially sourced Apis mellifera queen bees derived from a ‘Golden Italian’ strain obtained from Dewar Corp, located in Kalbar, Queensland, Australia. All hives were located at least 2 m apart and facing different directions.
In Sydney, 14 eight-frame Langstroth hives (thymol n = 7, control n = 7) were located at the Wheen Bee Foundation (33° 35′ 13.3″ S; 150° 43′ 32.5″ E), with each hive consisting of one box (‘full depth’ boxes). These hives were rented from the Wheen Bee Foundation in Richmond, New South Wales, Australia. The hives were previously established at unknown dates and populated with bee strains obtained from local beekeepers with no mention of a particular strain. All 14 hives were placed 4 m apart.
2.2 Treatments
Colonies in the treatment groups were treated with Apiguard® (Vita Europe Ltd.), a thymol-based acaricide that contains 12.5 g (25%) thymol (2-isopropyl-5-methylphenol) in a slow-release gelatine form that spreads within the hive through volatilisation (Floris et al. 2004; Mondet et al. 2011). The Apiguard® aluminium trays were donated by Plant Health Australia. We administered Apiguard® in autumn as per the manufacturer’s instructions by introducing one tray on top of the brood frames. Trays were replaced after 2 weeks. The second tray was also removed after 2 weeks (Vita Bee Health 2017).
2.3 Hygienic behaviour experiments
Two experiments were performed to test the impact of thymol on the hygienic performances of the hives: (1) the frozen brood assay to assess uncapping and brood removal (Spivak and Gilliam 1998a) and (2) the dead bee removal assay to assess the undertaking of dead bees (Visscher 1983). These assays were conducted in autumn 2017 from March 27 to May 10 in Hobart and from April 13 to May 25 in Sydney, on three different times per hive: once before thymol treatment and twice during the thymol exposure period with a two-week interval between assays (Figure 1).
Maximum and minimum temperatures and experimental timeline of the assays in Hobart and Sydney. Plain red lines represent the maximum temperatures and dashed blue lines the minimum temperatures (°C). Temperature values were obtained from the Australian Bureau of Meteorology for Hobart and Richmond from weather stations located less than 3 km from the apiaries in Hobart and Sydney.
The frozen brood assay is a standard assay used to evaluate brood removal (reviewed in Spivak and Gilliam 1998b). It involves freeze-killing honey bee brood using liquid nitrogen and assessing the proportion of killed brood that was uncapped and removed after 48 h (Spivak and Downey 1998). On day 0 of the experiment, an empty drawn frame was inserted into the centre of the brood chamber to allow the queen to lay a large patch of eggs, thereby obtaining a patch of capped cells of uniform brood age covering at least a quarter of the surface of the frame. On day 14, the previously inserted frame comb was extracted and assessed for the presence of capped brood. If the amount of capped brood proved insufficient, a different comb from within that hive was selected. A thin metal cylinder of 8 cm diameter was then partially inserted into the selected frame, on an area covered with capped brood. The number of capped and uncapped brood within the cylinder was then recorded by photographing the area enclosed by the cylinder on both sides of the frame using a Canon EOS 600 with a macro 18–135 mm Canon lens in Hobart and a Nikon D3000 with a Nikon 18–55 mm lens in Sydney. Three to four hundred millilitres of liquid nitrogen was then poured into the cylinder to kill the brood within the enclosed area and the frame was left to thaw before it was returned to the hive. A new, empty frame was again inserted into the centre of the hive instead of the fourth or fifth frame for the next frozen brood assessment on day 28. On day 16, 2 days after the liquid nitrogen treatment, the frame that had been frozen was removed, photographed and returned to the hive. Hives were ranked by the percentage of brood removal and allocated to control or thymol groups using a systematic allocation to ensure that hives with good and bad hygienic levels were equally distributed amongst each treatment group.
On day 21, an Apiguard® tray was placed as per the manufacturer’s instructions on top of the frames of each of the thymol treatment hives. The thymol trays were replaced on day 35. The frozen brood assays were repeated on days 28 and 42. Photographs were taken before freezing and 2 days after the brood was frozen as described above.
The impact of thymol on the removal of dead worker bees was assessed using a standard assay (Visscher 1983, Barron and Robinson 2005). Prior to the commencement of the experiment, 80 adult bees were collected from each hive by shaking live bees from hive combs into BugDorm-42,222 mesh cages in Hobart and into 50-mL plastic falcon tubes in Richmond. The bees were killed and stored by freezing at – 18 °C and marked with pink fluorescent powder (Day-Glo Colour Corp, Swift and Company Ltd., Ohio). This was done by putting a small amount of fluorescent powder into a plastic vial containing dead bees and gently shaking the vial until each bee was coated in powder.
On the day of the experiment, 20 marked frozen bees from each hive were thawed at room temperature for 1 hour. Whilst the dead bees were thawing, the external ground area at the front of each hive was overlaid with white cotton sheets (150 cm × 135 cm) to allow easier detection of the dead bees. Once thawed, the dead bees were emptied into each hive from which they were originally collected, onto the centre frames of the uppermost box of each hive. The time it took for undertaker bees to drag each dead bee out of the hive entrance was recorded to the nearest second with a stopwatch. The hive entrance was watched for 20 min following the addition of the dead bees. Any bees that were removed after 20 min were not included in the analysis. The dead bee assays were conducted on day 16, before the thymol treatment, and on days 30 and 44 during thymol treatment. Observations took place between 10 a.m. and 3 p.m. at both locations and the timing of the experiments was kept constant across assays.
2.4 Data handling and statistical analysis
All analyses were conducted using R v.3.3.1. The efficiency of hives at uncapping cells and removing dead brood and bees was analysed using mixed effects logistic regressions (GLMM) with the package lme4 v.1.1–14. The number of successes and failures at uncapping or removing dead individuals was used as a dependent variable whilst treatment, location and assay numbers were treated as independent variables. Hive number was added as a random factor. Post hoc comparisons between the treatment groups at each site and for each assay were obtained using the package lsmeans v.2.27–61. The time taken to remove the first dead bee out of the hive was analysed using Cox proportional hazards regressions (CPH) with the package survival v.2.41–3. To detect the effect of location, we built an initial statistical model with the time of removal from the hive as a dependent variable and the treatments, assay numbers, location and their interactions as independent variables and hive number was added as a cluster. To analyse the effect of treatments at each location, we built separate models for the two sites and used the time taken to remove the first bee as the dependent variable; treatments, assay numbers and their interactions as independent variables and hive number as a cluster. Standard errors were calculated as the standard deviation divided by the square root of the sample size. Figures were created using Excel v.1806 and the ggplot package for R. Data are given in Online Resource 1.
3 Results
3.1 Uncapping
In the first assay on day 14, before thymol treatment, the percentage of uncapping was significantly different between Hobart and Sydney (GLMM; z-ratio = 2.167, p = 0.03). Bees from Hobart uncapped 59.8 ± 1.9% (mean ± SE; Figure 2a) of the freeze-killed brood cells, whilst those from Sydney uncapped 73.9 ± 0.1% (Figure 2b). There were no differences in uncapping between the control and thymol treatment groups within each location (GLMM; Hobart: z-ratio = 0.29, p = 0.78; Sydney: z-ratio = − 0.23, p = 0.82). In the second assay on day 28, after thymol was introduced into the hives, thymol hives uncapped significantly more cells than the control hives in both Hobart and Sydney (GLMM; Hobart: z-ratio = − 2.49, p = 0.01; Sydney: z-ratio = − 3.27, p = 0.001). In Hobart, thymol hives uncapped 69.0 ± 9.2% of the freeze-killed brood cells and were more efficient at uncapping cells than control colonies (50.7 ± 9.7%) (Figure 2a) whereas in Sydney, thymol hives uncapped 87.4 ± 5.3% of the cells and were more efficient at uncapping cells than control hives (uncapped 71.0 ± 11.2%) (Figure 2b). In the third assay on day 42, after the thymol trays had been replaced, there were no differences in uncapping between the thymol and control groups at either location (GLMM; Hobart: z-ratio = − 1.55, p = 0.12; Sydney: z-ratio = 0.06, p = 0.95). In Hobart, 55.3 ± 2.9% of the cells were uncapped (Figure 2a), whilst in Sydney 58.1 ± 1.7% were uncapped (Figure 2b).
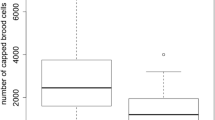
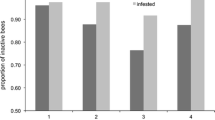
Mean percentage (± SE) of uncapping of freeze-killed brood in the European honey bee (Apis mellifera) prior to thymol treatment (assay 1) and during thymol treatment (assays 2 and 3) in a Hobart and b Sydney. Mean (± SE) percentage of freeze-killed brood prior to thymol (assay 1) and during thymol treatment (assays 2 and 3) in c Hobart and d Sydney. Mean (± SE) percentage removal of dead European honey bee adults from within control and thymol-treated hives prior to thymol treatment (assay 1) and during thymol treatment (assays 2 and 3) in e Hobart and f Sydney. All Hobart assays consisted of 16 hives and Sydney assays of 14 hives. Asterisks show statistical significance for p < 0.05.
3.2 Brood removal
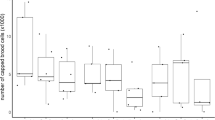
The percentages of brood removed in the first assay were similar in Hobart and Sydney (GLMM: z-ratio = 1.746, p = 0.08). Hives from Hobart removed 53.6 ± 4.2% of the brood (58.22 ± 10.32% in control hives and 50.35 ± 8.56% in thymol hives) (Figure 2c GLMM) and hives from Sydney removed 68.7 ± 1.0% of the brood (70.85 ± 7.41% in control hives and 67.33 ± 6.57% in thymol hives) (Figure 2d). There was no difference in brood removal between the control and thymol groups within each location (GLMM; Hobart: z-ratio = 0.99, p = 0.32; Sydney: z-ratio = 0.51, p = 0.61). In the second assay, after thymol was introduced into the hives, thymol-treated hives removed 84.1 ± 6.3% of the freeze-killed brood and were more efficient in removing dead brood than the control hives (66.2 ± 12.5%) in Sydney. In Hobart, hives removed 48.8 ± 5.3% but there was no significant difference between treatments (42.99 ± 9.28% in control hives and 53.66 ± 9.56% in thymol hives) (Figure 2d; GLMM; Sydney: z-ratio = − 2.85, p = 0.004; Hobart: z-ratio = − 1.52, p = 0.13). In the third assay, thymol-treated hives removed 49.2 ± 6% of the dead brood and were more efficient than the control hives (36.7 ± 5.6%) in Hobart, but there was no significant difference between treatments in Sydney where bees removed 34 ± 1% of the dead brood (53.25 ± 7.92% in control hives and 55.10 ± 8.80% in thymol hives) (Figure 2c; GLMM: Hobart: z-ratio = − 2.08, p = 0.04, Sydney: z-ratio = 0.29, p = 0.77). None of the hives removed more than 95% of the freeze-killed brood, the hive that performed the best removed 88% of the brood in Hobart (Figure 3).
3.3 Adult bee removal
The removal of dead adult honey bees varied between the two locations (Figure 4). During the first assay, the percentage of bees removed differed between Hobart and Sydney (GLMM: z-ratio = − 1.95, p = 0.05). Nevertheless, at both locations, the percentage of the dead adult honey bees removed was similar between the control and the thymol groups, in all three assays (GLMM: Hobart, assay 1: z-ratio = 0.64, p = 0.52, assay 2: z-ratio = − 1.49, p = 0.14, assay 3 z-ratio = 0.66, p = 0.51; Sydney, assay 1: z-ratio = − 0.98, p = 0.33, assay 2: z-ratio = 1.78, p = 0.07, assay 3: z-ratio = − 0.24, p = 0.81). In Hobart, the mean percentage of removal in assays 1, 2 and 3 were 66.9 ± 3.8%, 66.3 ± 6.9% and 62.2 ± 0.9% respectively (Figure 2e). In Sydney, hives removed 43.9 ± 2.5%, 43.2 ± 6.8% and 55.7 ± 2.9% in assays 1, 2 and 3 respectively (Figure 2f).
Mean rate of removal of dead honey bee adults in control hives prior to thymol (assay 1, continuous line) and during thymol treatment (assay 2, dashed and dotted line; assay 3, dashed line); in a Hobart and b Sydney. The mean rate of removal of dead honey bee adults in thymol hives prior to thymol and during thymol treatment in a Hobart (n = 16 hives) and b Sydney (n = 14 hives). There were no significant differences between treatment groups.
The time it took to remove the first dead bee differed between sites (CPH: z = 1.98, p = 0.049). Nevertheless, there was no difference in the time it took to remove the first dead bee from the hives between the control and the thymol groups at either location (CPH: Hobart: z = 0.43, p = 0.67; Sydney: z = − 0.43, p = 0.67), no effect between the assay numbers (CPH: Hobart: z = 0.29, p = 0.77; Sydney: z = − 0.13, p = 0.89) and no effect of the interaction between assay numbers and treatments (CPH: Hobart: z = − 0.09, p = 0.92; Sydney: z = − 0.71, p = 0.48). In Hobart, on average, the first dead bee was removed at 219 ± 34 s, 206 ± 14 s and 183 ± 13 s in assays 1, 2 and 3 respectively (Figure 5a). In Sydney, on average, the first bee was removed at 114 ± 1 s, 47 ± 7 s and 236 ± 108 s in assays 1, 2 and 3 respectively (Figure 5b).
Meantime (± SE) required for the removal of the first dead adult honey bee in seconds in control and thymol-treated hives prior to thymol (assay 1) and during thymol treatment (assay 2 and 3) in a Hobart (n = 16 hives) and b Sydney (n = 14 hives). There were no significant differences between treatment groups.
4 Discussion
We assessed the effects of thymol on three essential hygienic behaviours of the European honey bee. Bees treated with thymol uncapped more cells after the first thymol tray was introduced but not after the second tray was introduced. Bees treated with thymol removed more dead brood than bees from the control group after the first thymol tray was introduced in Sydney but not after it was replaced, and this effect was only found after the tray was replaced in Hobart. Bees treated with thymol were not significantly faster at removing dead adult bees and did not remove significantly more dead adults from the hives. The differences between the effects of thymol on the removal and uncapping of dead brood and on the removal of dead adults suggests that these behaviours could be independent or triggered by different cues.
We have shown that thymol improved the uncapping and removal of brood that were freeze-killed. However, thymol has also been reported to decrease global brood amounts in honey bee colonies (Tananaki et al. 2014) and to cause the removal of live brood located near thymol containers (Baggio et al. 2004; Marchetti et al. 1984; Imdorf et al. 1999). Altogether, these results indicate that thymol could cause increased brood removal regardless of their health. Negative effects of miticide treatments on brood development have also been reported for formic acid treatments (Bolli et al. 1993).
The effects of thymol were not consistent throughout the duration of the treatment, and uncapping was only improved in the second assay in Sydney and in the second assay in Hobart. Similarly, thymol increased the removal of dead brood in the second assay at both locations, but not in the third assay. This suggests that the effects of the thymol treatment on bees change over the course of treatment. It has previously been proposed that bees could become habituated to thymol, which could explain why thymol had limited effects on the hygienic behaviours of the bees in the third assay (Mondet et al. 2011).
The efficiency of bees at performing these three hygienic behaviours was not consistent between locations. Bees removed more dead adult bees in Hobart than in Sydney but uncapped and removed more dead brood in Sydney than in Hobart. The hives used in Hobart and Sydney differed in shape and volume, and workers and brood may have been exposed to different concentrations of thymol, which could explain these variations. Major environmental differences between Hobart and Sydney, separated by a thousand kilometres and 9° of latitude, could explain these results. Environmental interactions have previously been suggested to explain inconsistent results in studies that show both positive and negative effects of pesticides, including thymol, on honey bee colonies (Sammataro et al. 2004; Lodesani and Costa 2008; Woodcock et al. 2017). Genetic differences may also modulate hygienic levels and the effect of thymol on hygienic behaviours between locations. The queens used in Hobart were obtained from a commercial queen breeder, but the origin of queens used in Sydney was unknown. Honeybee breeds that are more efficient at performing certain hygienic behaviours can be artificially selected (Spivak and Reuter 2001a; Spivak and Reuter 2001b), but to our knowledge, none of the strains of honey bees used in this experiment were selected for hygienic behaviours. None of the hives used in this experiment removed more than 95% of the dead brood before the thymol treatment, which is the usual threshold used to classify colonies as hygienic (Spivak and Reuter 1998; Bigio et al. 2013). Further research is thus needed to investigate how thymol affects hygienic behaviour in hygienic honey bee strains.
These results highlight the need to replicate experiments in different environments when assessing the effect of pesticides on honey bees. Further studies should focus on how the impact of thymol and other miticides observed on honey bee behaviours affect the survival and productivity of the entire honey bee colonies.
References
Baggio, A., Arculeo, P., Nanetti, A., Marinelli, E., Mutinelli, F. (2004) Field trials with different thymol-based products for the control of varoosis. Am. Bee J. 144 (5), 395–400.
Barron, A.B., Robinson, G.E. (2005). Selective modulation of task performance by octopamine in honey bee (Apis mellifera) division of labour. J. Comp. Physiol. A 191 (7), 659–668.
Berry, J.A., Hood, W.M., Pietravalle, S., Delaplane, K.S. (2013) Field-level sublethal effects of approved bee hive chemicals on honey bees (Apis mellifera L). PloS One 8 (10), e76536.
Bigio, G., Schürch, R., Ratnieks, F. L. (2013) Hygienic behavior in honey bees (Hymenoptera: Apidae): effects of brood, food, and time of the year. J. Econ. Entomol. 106 (6), 2280–2285.
Boecking, O., Spivak, M. (1999) Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 30 (2–3), 141–158.
Boi, M., Serra, G., Colombo, R., Lodesani, M., Massi, S. and Costa, C. (2016) A 10 year survey of acaricide residues in beeswax analysed in Italy. Pest. Manag. Sci., 72 (7), 1366–1372.
Bolli, H.K., Bogdanov, S., Imdorf, A., Fluri, P. (1993) Zur Wirkungsweise von Ameisensäure bei Varroa jacobsoni Oud und der Honigbiene (Apis mellifera L). Apidologie, 24 (1), 51–57.
Charpentier, G., Vidau, C., Ferdy, J.-B. , Tabart, J., Vetillard, A. (2014) Lethal and sub-lethal effects of thymol on honeybee (Apis mellifera) larvae reared in vitro. Pest. Manag. Sci. 70 (1), 140–147.
Choe, D.H., Millar, J.G., Rust, M.K. (2009) Chemical signals associated with life inhibit necrophoresis in Argentine ants. Proc. Natl. Acad. Sci. 106 (20), 8251–8255.
Coffey, M.F. (2007) Biotechnical methods in colony management, and the use of Apiguard (R) and Exomite (TM) Apis for the control of the varroa mite (Varroa destructor) in Irish honey bee (Apis mellifera) colonies. J. Apic. Res. 46 (4), 213–219.
Coffey, M.F., Breen, J. (2013) Efficacy of Apilife Var® and Thymovar® against Varroa destructor as an autumn treatment in a cool climate. J. Apic. Res. 52 (5), 210–218.
Colin, M., de Lahitte, J.D., Larribau, E., Boué, T. (1989) Activité des huiles essentielles de Labiées sur Ascophaera apis et traitement d'un rucher. Apidologie 20 (3), 221–228.
Costa, C., Lodesani, M., Maistrello, L. (2010) Effect of thymol and resveratrol administered with candy or syrup on the development of Nosema ceranae and on the longevity of honeybees (Apis mellifera L.) in laboratory conditions. Apidologie 41 (2), 141–150.
Evans, J.D., Spivak, M. (2010) Socialized medicine: individual and communal disease barriers in honey bees. J. Invert. Path. 103 Suppl 1, S62-S72.
Faucon, J.P., Drajnudel, P., Fléché, C. (1995) Mise en évidence d’une diminution de l’efficacité de l’Apistan® utilisé contre la varroose de l’abeille (Apis mellifera L). Apidologie 26 (4), 291–295.
Floris, I., Satta, A., Cabras, P., Garau, V.L., Angioni, A. (2004) Comparison between two thymol formulations in the control of Varroa destructor: effectiveness, persistence, and residues. J Econ. Entomol. 97 (2), 187–191.
Gerdts, J. (2014) Hygienic Behaviour in the Australian Queen Bee Breeding Program, Australian Honey Bee Industry Council, Victoria.
Goodwin, M., Van Eaton, C. (2001) Control of varroa. A guide for New Zealand Beekeepers. New Zealand Ministry of Agriculture and Forestry (MAF). Wellington.
Gordon, D.M. (1983) Dependence of necrophoric response to oleic acid on social context in the ant, Pogonomyrmex badius. J. Chem. Ecol. 9 (1), 105–111.
Gramacho, K.P., Spivak, M. (2003) Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior. Behav. Ecol. Sociobiol. 54 (5), 472–479.
Grobov, O., Mikitiuk, V., Guseva, L. (1981) Thymol, a substance with a wide spectrum of action. Apiacta 2, 64–66.
Guzmán-Novoa, E., Eccles, L., Calvete, Y., Mcgowan, J., Kelly, P.G., Correa-Benítez, A. (2010) Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 41 (4), 443–450.
Haarmann, T., Spivak, M., Weaver, D., Weaver, B., Glenn, T. (2002) Effects of fluvalinate and coumaphos on queen honey bees (Hymenoptera: Apidae) in two commercial queen rearing operations. J Econ Entomol 95 (1), 28–35.
Hafi, A., Millist, N., Morey, K., Caley, P., Buetre, B. (2012) A benefit-cost framework for responding to an incursion of Varroa destructor. National Biosecurity Committee, Canberra.
Imdorf, A., Bogdanov, S., Ochoa, R.I., Calderone, N.W. (1999) Use of essential oils for the control of Varroa jacobsoni Oud. in honey bee colonies. Apidologie 30 (2–3), 209–228.
Iwasaki, J.M., Barratt, B.I., Lord, J.M., Mercer, A.R., Dickinson, K.J. (2015) The New Zealand experience of varroa invasion highlights research opportunities for Australia. Ambio 44 (7), 694–704.
Jong, D.D., Morse, R.A., Eickwort, G.C. (1982) Mite pests of honey bees. Ann. Rev. Ent. 27 (1), 229–252.
Kanga, L.H., Adamczyk, J., Marshall, K., Cox, R. (2010) Monitoring for resistance to organophosphorus and pyrethroid insecticides in Varroa mite populations. J Econ. Entomol. 103 (5), 1797–1802.
Lapidge, K.L., Oldroyd, B.P., Spivak, M. (2002) Seven suggestive quantitative trait loci influence hygienic behavior of honey bees. Naturwissenschaften 89 (12), 565–568.
Le Conte, Y., Ellis, M., Ritter, W. (2010) Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41 (3), 353–363.
Lodesani, M., Costa, C. (2008) Maximizing the efficacy of a thymol based product against the mite Varroa destructor by increasing the air space in the hive. J. Apic. Res. 47 (2), 113–117.
Loucif-Ayad, W., Aribi, N., Smagghe, G., Soltani, N. (2010) A scientific note on the impact of acaracides on the nutritional biochemistry of Apis mellifera intermissa (Hymenoptera: Apidae). Apidologie 41 (2), 135–137.
Marchetti, S., Barbattini, R., D'Agaru, M. (1984) Comp. effectiveness of treatments used to control Varroa jacobsoni Oud. Apidologie 15 (4), 363–378.
Masterman, R., Smith, B.H., Spivak, M. (2000) Brood odor discrimination abilities in hygienic honey bees (Apis mellifera L.) using proboscis extension reflex conditioning. J. Insect Behav. 13 (1), 87–101.
McAfee, A., Chapman, A., Iovinella, I., Gallagher-Kurtzke, Y., Collins, T. F., et al. (2018) A death pheromone, oleic acid, triggers hygienic behavior in honey bees (Apis mellifera L.). Sci. Rep. 8 (1), 5719.
Milani, N. (1995) The resistance of Varroa jacobsoni Oud. to pyrethroids: a laboratory assay. Apidologie 26 (5), 415–429.
Milani, N. (1999) The resistance of Varroa jacobsoni Oud. to acaricides. Apidologie 30 (2–3), 229–234.
Mondet, F., Goodwin, M., Mercer, A. (2011) Age-related changes in the behavioural response of honeybees to Apiguard®, a thymol-based treatment used to control the mite Varroa destructor. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 197 (11), 1055–1062.
Mondet, F., Alaux, C., Severac, D., Rohmer, M., Mercer, A.R., et al. (2015) Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci. Rep. 5, 10454.
Peng, Y.-S., Fang, Y., Xu, S., Ge, L. (1987) The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. J. Invert. Path. 49 (1), 54–60.
Price, K.L., Lummis, S.C. (2014) An atypical residue in the pore of Varroa destructor GABA-activated RDL receptors affects picrotoxin block and thymol modulation. Insect Biochem. Molec. Biol. 55 (1), 19–25.
Rath, W. (1999) Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie 30 (2–3), 97–110.
Rath, W., Drescher, W. (1990) Response of Apis cerana Fabr towards brood infested with Varroa jacobsoni Oud and infestation rate of colonies in Thailand. Apidologie 21 (4), 311–321.
Ratnieks, F.L., Carreck, N.L. (2010) Clarity on honey bee collapse? Science 327 (5962), 152–153.
Robinson, G.E., Page Jr, R.E. (1988) Genetic determination of guarding and undertaking in honey-bee colonies. Nature, 333 (6171), 356.
Rosenkranz, P., Aumeier, P., Ziegelmann, B. (2010) Biology and control of Varroa destructor. J. Invert. Path. 103, S96-S119.
Sammataro, D., Hoffman, G.D., Wardell, G., Finley, J., Ostiguy, N. (2004) Testing a combination of control tactics to manage Varroa destructor (Acari: Varroidae) population levels in honey bee (Hymenoptera: Apidae) colonies. Int. J. Acarol. 30 (1), 71–76.
Škerl, M.I.S., Kmecl, V., Gregorc, A. (2010) Exposure to pesticides at sublethal level and their distribution within a honey bee (Apis mellifera) colony. Bull. Env. Contam. Toxicol. 85 (2), 125–128.
Spivak, M. (1996) Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie 27 (4), 245–260.
Spivak, M., Downey, D.L. (1998) Field assays for hygienic behavior in honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 91 (1), 64–70.
Spivak, M., Gilliam, M. (1993) Facultative expression of hygienic behaviour of honey bees in relation to disease resistance. J. Apic. Res. 32 (3–4), 147–157.
Spivak, M., Gilliam, M. (1998a) Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa - Part II. Studies on hygienic behaviour since the Rothenbuhler era. Bee world 79 (4), 169–186.
Spivak, M., Gilliam, M. (1998b) Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa Part I. Hygienic behaviour and resistance to American foulbrood. Bee world 79 (3), 124–134.
Spivak, M., Reuter G.S. (1998) Honey bee hygienic behavior. Am. Bee J. 138 (4): 283–286.
Spivak, M., Reuter, G.S. (2001a) Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 32 (6), 555–565.
Spivak, M., Reuter, G.S. (2001b) Varroa destructor infestation in untreated honey bee (Hymenoptera: Apidae) colonies selected for hygienic behavior. J. Econ. Entomol. 94 (2), 326–331.
Tananaki, C., Goras, G., Huggett, N., Karazafiris, E., Dimou, M., et al. (2014) Evaluation of the impact of Exomite Pro™ on Varroa mite (Varroa destructor) populations and honeybee (Apis mellifera) colonies: efficacy, side effects and residues. Parasitol. Res. 113 (4), 1251–1259.
Trumbo, S.T., Robinson, G.E. (1997) Learning and Task Interference by Corpse-removal Specialists in Honey Bee Colonies. Ethology 103 (11), 966–975.
Trumbo, S.T., Huang, Z.Y., Robinson, G.E. (1997) Division of labor between undertaker specialists and other middle-aged workers in honey bee colonies. Behav. Ecol. Sociobiol. 41 (3), 151–163.
Visscher, P.K. (1983) The honey bee way of death: Necrophoric behaviour in Apis mellifera colonies. Anim. Behav. 31 (4), 1070–1076.
Vita Bee Health (2017) Apiguard: Varroa Control, Vita Europe Limited, London.
Wallner, K., Fries, I. (2003) Control of the mite Varroa destructor in honey bee colonies. Pestic. Outlook 14 (2), 80–84.
Wilson, E.O., Durlach, N.I., Roth, L.M. (1958) Chemical releasers of necrophoric behavior in ants. Psyche 65 (4), 108–114.
Woodcock, B., Bullock, J., Shore, R., Heard, M., Pereira, M., et al. (2017) Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 356 (6345), 1393–1395.
Acknowledgements
We thank Plant Health Australia for donating the Apiguard® thymol trays required for this study. We thank the Wheen Bee Foundation for use of the Foundation’s research apiary at Richmond NSW. We thank Ryan Warren, Hui Meng Law, Elyna Tong, Casey Davies and Rebekah Smart for their technical help with data collection in Hobart. We thank Jack Westacott for his technical help with data collection in Sydney. MYL received a scholarship from the Fruit Growers Tasmania. We thank two anonymous reviewers for their useful comments on the manuscript.
Funding
ABB and TC were supported by the Lord Mayor’s Charitable Foundation/Eldon & Anne Foote Trust. TC was funded by an iMQRES scholarship.
Author information
Authors and Affiliations
Contributions
All authors were involved in conceiving this research, designing and performing the experiments and analysing the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
Additional information
Manuscript editor: Peter Rosenkranz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Effets du thymol sur le comportement hygiénique des abeilles européennes
Acaricide / Apis mellifera / thymol
Auswirkungen von Thymol auf das Hygieneverhalten europäischer Honigbienen
Akarizid / Apis mellifera / Thymol
Electronic supplementary material
ESM 1.
(XLSX 32 kb)
Rights and permissions
About this article
Cite this article
Colin, T., Lim, M.Y., Quarrell, S.R. et al. Effects of thymol on European honey bee hygienic behaviour. Apidologie 50, 141–152 (2019). https://doi.org/10.1007/s13592-018-0625-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-018-0625-8