Abstract
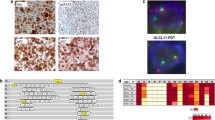
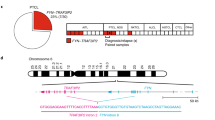
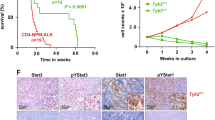
TK-ALCL1, a novel anaplastic lymphoma kinase (ALK)-positive anaplastic large-cell lymphoma (ALK+ ALCL) cell line, was established from the primary tumor site of a 59-year-old Japanese male patient. The immune profile of TK-ALCL1 corresponds to that seen typically in primary ALCL cells, i.e., positive for ALK, CD30, EMA, and CD4, but negative for CD2, CD3, CD5, CD8a, and EBV-related antigens. The rearrangement of the T cell receptor-gamma locus shows that TK-ALCL1 is clonally derived from T-lineage lymphoid cells. FISH and RT-PCR analysis revealed that TK-ALCL1 has the nucleophosmin (NPM)-ALK fusion transcript, which is typical for ALK+ ALCL cell lines. When TK-ALCL1 was subcutaneously inoculated into 6-week-old BALB/c Rag2−/−/Jak3−/− (BRJ) mice, it formed tumor masses within 4–6 weeks. Morphological, immunohistochemical, and molecular genetic investigations confirmed that the xenograft and the original ALCL tumor were identical. The ALK inhibitors Alectinib and Lorlatinib suppressed proliferation in a dose-dependent manner. Thus, TK-ALCL1 provides a useful in vitro and in vivo model for investigation of the biology of ALK+ ALCL and of novel therapeutic approaches targeting ALK.





Similar content being viewed by others
Data availability
The data of this cell line are not applicable.
References
Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848–58.
Morris SW, Kirstein MN, Valentine MB, Dittmer K, Shapiro DN, Look AT, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1995;267(5196):316–7. https://doi.org/10.1126/science.267.5196.316-b.
Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–48. https://doi.org/10.1038/s41375-022-01620-2.
Wu R, Lim MS. Updates in pathobiological aspects of anaplastic large cell lymphoma. Front Oncol. 2023;13:1241532. https://doi.org/10.3389/fonc.2023.1241532.
Irshaid L, Xu ML. ALCL by any other name: the many facets of anaplastic large cell lymphoma. Pathology. 2020;52(1):100–10. https://doi.org/10.1016/j.pathol.2019.09.007.
Shustov A, Soma L. Anaplastic large cell lymphoma: contemporary concepts and optimal management. Cancer Treat Res. 2019;176:127–44. https://doi.org/10.1007/978-3-319-99716-2_6.
Zhang XR, Chien PN, Nam SY, Heo CY. Anaplastic large cell lymphoma: molecular pathogenesis and treatment. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14071650.
Berger GK, McBride A, Lawson S, Royball K, Yun S, Gee K, et al. Brentuximab vedotin for treatment of non-Hodgkin lymphomas: a systematic review. Crit Rev Oncol Hematol. 2017;109:42–50. https://doi.org/10.1016/j.critrevonc.2016.11.009.
Fukano R, Mori T, Sekimizu M, Choi I, Kada A, Saito AM, et al. Alectinib for relapsed or refractory anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: an open-label phase II trial. Cancer Sci. 2020;111(12):4540–7. https://doi.org/10.1111/cas.14671.
Nambirajan A, Jain D. Cell blocks in cytopathology: an update. Cytopathology. 2018;29(6):505–24. https://doi.org/10.1111/cyt.12627.
Katano H, Hoshino Y, Morishita Y, Nakamura T, Satoh H, Iwamoto A, et al. Establishing and characterizing a CD30-positive cell line harboring HHV-8 from a primary effusion lymphoma. J Med Virol. 1999;58(4):394–401.
Shiota M, Fujimoto J, Takenaga M, Satoh H, Ichinohasama R, Abe M, et al. Diagnosis of t(2;5)(p23;q35)-associated Ki-1 lymphoma with immunohistochemistry. Blood. 1994;84(11):3648–52. https://doi.org/10.1182/blood.V84.11.3648.bloodjournal84113648.
Okada S, Vaeteewoottacharn K, Kariya R. Application of highly immunocompromised mice for the establishment of patient-derived xenograft (PDX) models. Cells. 2019. https://doi.org/10.3390/cells8080889.
Ono A, Hattori S, Kariya R, Iwanaga S, Taura M, Harada H, et al. Comparative study of human hematopoietic cell engraftment into BALB/c and C57BL/6 strain of rag-2/jak3 double-deficient mice. J Biomed Biotechnol. 2011;2011: 539748. https://doi.org/10.1155/2011/539748.
Pineiro-Yanez E, Reboiro-Jato M, Gomez-Lopez G, Perales-Paton J, Troule K, Rodriguez JM, et al. PanDrugs: a novel method to prioritize anticancer drug treatments according to individual genomic data. Genome Med. 2018;10(1):41. https://doi.org/10.1186/s13073-018-0546-1.
Robin T, Capes-Davis A, Bairoch A. CLASTR: the cellosaurus STR similarity search tool—a precious help for cell line authentication. Int J Cancer. 2020;146(5):1299–306. https://doi.org/10.1002/ijc.32639.
Stein H, Foss HD, Durkop H, Marafioti T, Delsol G, Pulford K, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96(12):3681–95.
Muzzafar T, Wei EX, Lin P, Medeiros LJ, Jorgensen JL. Flow cytometric immunophenotyping of anaplastic large cell lymphoma. Arch Pathol Lab Med. 2009;133(1):49–56. https://doi.org/10.5858/133.1.49.
Medeiros LJ, Elenitoba-Johnson KS. Anaplastic large cell lymphoma. Am J Clin Pathol. 2007;127(5):707–22. https://doi.org/10.1309/r2q9ccuvtlrycf3h.
van Dongen JJ, van der Burg M, Langerak AW. Split-signal FISH for detection of chromosome aberrations. Hematology. 2005;10(Suppl 1):66–72. https://doi.org/10.1080/10245330512331389980.
Drexler HG, MacLeod RA. Malignant hematopoietic cell lines: in vitro models for the study of anaplastic large-cell lymphoma. Leukemia. 2004;18(10):1569–71. https://doi.org/10.1038/sj.leu.2403465.
Bairoch A. The cellosaurus, a cell-line knowledge resource. J Biomol Tech. 2018;29(2):25–38. https://doi.org/10.7171/jbt.18-2902-002.
Epstein AL, Kaplan HS. Biology of the human malignant lymphomas. I. Establishment in continuous cell culture and heterotransplantation of diffuse histiocytic lymphomas. Cancer. 1974;34(6):1851–72. https://doi.org/10.1002/1097-0142(197412)34:6%3c1851::aid-cncr2820340602%3e3.0.co;2-4.
Fischer P, Nacheva E, Mason DY, Sherrington PD, Hoyle C, Hayhoe FG, et al. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin’s lymphoma, showing a 2;5 translocation and rearrangement of the T cell receptor beta-chain gene. Blood. 1988;72(1):234–40.
Su IJ, Balk SP, Kadin ME. Molecular basis for the aberrant expression of T cell antigens in postthymic T cell malignancies. Am J Pathol. 1988;132(2):192–8.
Morgan R, Smith SD, Hecht BK, Christy V, Mellentin JD, Warnke R, et al. Lack of involvement of the C-Fms and N-Myc genes by chromosomal translocation T(2–5)(P23–Q35) common to malignancies with features of so-called malignant histiocytosis. Blood. 1989;73(8):2155–64.
Barbey S, Gogusev J, Mouly H, Le Pelletier O, Smith W, Richard S, et al. DEL cell line: a “malignant histiocytosis” CD30+ t(5;6)(q35;p21) cell line. Int J Cancer. 1990;45(3):546–53. https://doi.org/10.1002/ijc.2910450329.
Kadin ME, C-CMW SN, Fletcher JA, Morton CC, Pastuzak W, Rezuke W, Altman AJ. Childhood Ki-1+ anaplastic large cell lymphoma: establishment and characterization of a new tumor cell line transplantable to SCID mice. Blood. 1990;76:354.
Umiel T, Rettenmier CW, Siegel S, Ota S, Shimada H, Tran TW, et al. Establishment and characterization of a human mixed-lineage, T lymphoid myeloid cell-line (Usp-91). Blood. 1993;82(6):1829–37.
Shimakage M, Dezawa T, Tamura S, Tabata T, Aoyagi N, Koike M, et al. A Ki-1-positive cell line expressing Epstein-Barr virus antigens, established from a child with Ki-1-positive lymphoma. Intervirology. 1993;36(4):215–24. https://doi.org/10.1159/000150340.
Shiota M, Fujimoto J, Semba T, Satoh H, Yamamoto T, Mori S. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, AMS3. Oncogene. 1994;9(6):1567–74.
Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281–4. https://doi.org/10.1126/science.8122112.
Al-Hashmi I, Decoteau J, Gruss HJ, Zielenska M, Thorner P, Poon A, et al. Establishment of a cytokine-producing anaplastic large-cell lymphoma cell line containing the t(2;5) translocation: potential role of cytokines in clinical manifestations. Leuk Lymphoma. 2001;40(5–6):599–611. https://doi.org/10.3109/10428190109097658.
Merz H, Lange K, Gaiser T, Muller A, Kapp U, Bittner C, et al. Characterization of a novel human anaplastic large cell lymphoma cell line tumorigenic in SCID mice. Leuk Lymphoma. 2002;43(1):165–72. https://doi.org/10.1080/10428190210193.
Lamant L, Espinos E, Duplantier M, Dastugue N, Robert A, Allouche M, et al. Establishment of a novel anaplastic large-cell lymphoma-cell line (COST) from a ‘small-cell variant’ of ALCL. Leukemia. 2004;18(10):1693–8. https://doi.org/10.1038/sj.leu.2403464.
Thielen C, Bisig B, Gofflot S, Herens C, Fillet G, Jamar M, et al. CHIC cells: a novel ALK plus cell line derived from a relapsed anaplastic large cell lymphoma. Br J Haematol. 2011;152(3):356–60. https://doi.org/10.1111/j.1365-2141.2010.08414.x.
Bonzheim I, Geissinger E, Roth S, Zettl A, Marx A, Rosenwald A, et al. Anaplastic large cell lymphomas lack the expression of T cell receptor molecules or molecules of proximal T cell receptor signaling. Blood. 2004;104(10):3358–60. https://doi.org/10.1182/blood-2004-03-1037.
Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560–72. https://doi.org/10.1111/bjh.13958.
Iwahara T, Fujimoto J, Wen DZ, Cupples R, Bucay N, Arakawa T, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14(4):439–49. https://doi.org/10.1038/sj.onc.1200849.
Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui XL, et al. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene. 1997;14(18):2175–88. https://doi.org/10.1038/sj.onc.1201062.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6. https://doi.org/10.1038/nature05945.
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. https://doi.org/10.1056/NEJMoa1006448.
Roskoski R Jr. Anaplastic lymphoma kinase (ALK) inhibitors in the treatment of ALK-driven lung cancers. Pharmacol Res. 2017;117:343–56. https://doi.org/10.1016/j.phrs.2017.01.007.
Ferreri AJ, Govi S, Pileri SA, Savage KJ. Anaplastic large cell lymphoma, ALK-positive. Crit Rev Oncol Hematol. 2012;83(2):293–302. https://doi.org/10.1016/j.critrevonc.2012.02.005.
Gromowsky MJ, D’Angelo CR, Lunning MA, Armitage JO. ALK-positive anaplastic large cell lymphoma in adults. Fac Rev. 2023;12:21. https://doi.org/10.12703/r/12-21.
Brugieres L, Cozic N, Houot R, Rigaud C, Sibon D, Arfi-Rouche J, et al. Efficacy and safety of crizotinib in ALK-positive systemic anaplastic large-cell lymphoma in children, adolescents, and adult patients: results of the French AcSe-crizotinib trial. Eur J Cancer. 2023;191: 112984. https://doi.org/10.1016/j.ejca.2023.112984.
Kong XT, Pan PC, Sun HY, Xia HG, Wang XW, Li YY, et al. Drug discovery targeting anaplastic lymphoma kinase (ALK). J Med Chem. 2019;62(24):10927–54. https://doi.org/10.1021/acs.jmedchem.9b00446.
Debelenko LV, Arthur DC, Pack SD, Helman LJ, Schrump DS, Tsokos M. Identification of CARS-ALK fusion in primary and metastatic lesions of an inflammatory myofibroblastic tumor. Lab Invest. 2003;83(9):1255–65. https://doi.org/10.1097/01.lab.0000088856.49388.ea.
Gascoyne RD, Lamant L, Martin-Subero JI, Lestou VS, Harris NL, Muller-Hermelink HK, et al. ALK-positive diffuse large B-cell lymphoma is associated with Clathrin-ALK rearrangements: report of 6 cases. Blood. 2003;102(7):2568–73. https://doi.org/10.1182/blood-2003-03-0786.
Acknowledgements
The authors would like to thank the staff of Tochigi Cancer Center operation room for their support in handling the samples, Ms. Sawako Fujikawa for technical assistance, and Ms. Michiko Teramoto for secretarial assistance.
Funding
This research was funded by Strategic Core Technology Advancement Program (Supporting Industry Program) from Ministry of Economy, Trade and Industry, Japan.
Author information
Authors and Affiliations
Contributions
Conceptualization, K.K. and S.O.; methodology, P.S., J.P., R.K., and S.O.; software, P.S.; validation, P.S., J.P., Y.F., and S.O.; formal analysis, P.S., Y.S, R.T., T.O., and H.Y.; investigation, P.S., Y.F., S.O.; resources, S.K., R.N., T.H., Y. O., M. A., and K.H.; data curation, R.N.; writing—original draft preparation, P.S. and J.P.; writing—review and editing, K.K. and S.O.; visualization, P.S., Y.O., M.A., K.H., and S.O.; supervision, K.K., K.H., and S.O.; project administration, K.K. and S.O.; funding acquisition, S.O. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
The use of clinical materials for this study was approved by the Institutional Review Board of Tochigi Cancer Center and the Faculty of Life Science Kumamoto University Ethics Committee (30-78-20-2020119). Informed consent was obtained from the donor. Note: TK-ALCL1 is available from corresponding author upon request. The authors are planning to deposit TK-ALCL1 to Japanese Collection of Research Bioresources (JCRB) cell bank (Osaka, Japan).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sungwan, P., Panaampon, J., Kariya, R. et al. Establishment and characterization of TK-ALCL1: a novel NPM-ALK-positive anaplastic large-cell lymphoma cell line. Human Cell (2024). https://doi.org/10.1007/s13577-024-01077-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13577-024-01077-8