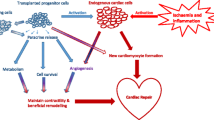
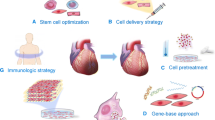
Abstract
Due to aging populations and changes in lifestyle, cardiovascular diseases including cardiomyopathy, hypertension, and atherosclerosis, are the leading causes of death worldwide. The heart is a complicated organ composed of multicellular types, including cardiomyocytes, fibroblasts, endothelial cells, vascular smooth muscle cells, and immune cells. Cellular specialization and complex interplay between different cell types are crucial for the cardiac tissue homeostasis and coordinated function of the heart. Mounting studies have demonstrated that dysfunctional cells and disordered cardiac microenvironment are closely associated with the pathogenesis of various cardiovascular diseases. In this paper, we discuss the composition and the homeostasis of cardiac tissues, and focus on the role of cardiac environment and underlying molecular mechanisms in various cardiovascular diseases. Besides, we elucidate the novel treatment for cardiovascular diseases, including stem cell therapy and targeted therapy. Clarification of these issues may provide novel insights into the prevention and potential targets for cardiovascular diseases.




Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- IVIG:
-
Intravenous immunoglobulin
- ECM:
-
Extracellular matrix
- PDGFRα:
-
Platelet-derived growth factor receptor α
- Ang II:
-
Angiotensin II
- EndoMT:
-
Endothelial-to-mesenchymal transition
- NO:
-
Nitric Oxide
- ET-1:
-
Endothelin-1
- iNOS:
-
Inducible nitric oxide synthase
- HCM:
-
Hypertrophic cardiomyopathy
- DCM:
-
Dilated cardiomyopathy
- RCM:
-
Restrictive cardiomyopathy
- ACM:
-
Arrhythmia Cardiomyopathy
- SR:
-
Sarcoplasmic reticulum
- RYR2:
-
Ryanodine receptor 2
- SERCA:
-
Sarco/endoplasmic reticulum Ca2+-ATPase
- cTnC:
-
Cardiac troponin C
- FGF2:
-
Fibroblast growth factor 2
- PDGF:
-
Platelet-derived growth factor
- HF:
-
Heart failure
- eNOS:
-
Endothelial nitric oxide synthase
- ROS:
-
Reactive oxygen species
- MI:
-
Myocardial infarction
- MMPs:
-
Matrix metalloproteinases
- DCs:
-
Dendritic cells
- MSCs:
-
Mesenchymal stem cells
- HSCs:
-
Hematopoietic stem cells
- EPCs:
-
Endothelial progenitor cells
- CSCs:
-
Cardiac stem cells
- iPSCs:
-
Embryonic stem cells and induced pluripotent stem cells
- bFGF:
-
Basic fibroblast growth factor
- VEGF:
-
Vascular endothelial growth factor
- LVEF:
-
Left ventricular ejection fraction
- AdMSC:
-
Adipose-derived mesenchymal stem cell
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
- hESCs:
-
Human embryonic stem cells
- CAR-T:
-
Chimeric antigen receptor T cell
- NHEJ:
-
Non-homologous terminal ligation
- HDR:
-
Homologous recombinant repair
- MYH6:
-
Myosin heavy chain 6
- MYBPC3:
-
Myosin-binding protein C3
- AAV:
-
Adeno-associated virus
References
Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145–58.
Takeda N, Manabe I. Cellular interplay between cardiomyocytes and nonmyocytes in cardiac remodeling. Int J Inflam. 2011;2011: 535241.
Aggarwal M, Aggarwal B, Rao J. Integrative medicine for cardiovascular disease and prevention. Med Clin North Am. 2017;101:895–923.
Mishra S, Chatterjee S. Lactosylceramide promotes hypertrophy through ROS generation and activation of ERK1/2 in cardiomyocytes. Glycobiology. 2014;24:518–31.
Liu T, Wen H, Li H, et al. Oleic acid attenuates Ang II (Angiotensin II)-induced cardiac remodeling by inhibiting FGF23 (Fibroblast Growth Factor 23) expression in mice. Hypertension. 2020;75:680–92.
Gruver CL, DeMayo F, Goldstein MA, Means AR. Targeted developmental overexpression of calmodulin induces proliferative and hypertrophic growth of cardiomyocytes in transgenic mice. Endocrinology. 1993;133:376–88.
Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–35.
Hirose K, Payumo AY, Cutie S, et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science. 2019;364:184–8.
Liu S, Tang L, Zhao X, et al. Yap promotes noncanonical wnt signals from cardiomyocytes for heart regeneration. Circ Res. 2021;129:782–97.
Frias MA, Pedretti S, Hacking D, et al. HDL protects against ischemia reperfusion injury by preserving mitochondrial integrity. Atherosclerosis. 2013;228:110–6.
Shende P, Xu L, Morandi C, et al. Cardiac mTOR complex 2 preserves ventricular function in pressure-overload hypertrophy. Cardiovasc Res. 2016;109:103–14.
Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51.
Tallquist MD. Cardiac fibroblast diversity. Annu Rev Physiol. 2020;82:63–78.
Yokota T, McCourt J, Ma F, et al. Type V collagen in scar tissue regulates the size of scar after heart injury. Cell. 2020;182:545-62.e23.
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801.
Ivey MJ, Kuwabara JT, Riggsbee KL, Tallquist MD. Platelet-derived growth factor receptor-α is essential for cardiac fibroblast survival. Am J Physiol Heart Circ Physiol. 2019;317:H330–44.
Jang SW, Ihm SH, Choo EH, et al. Imatinib mesylate attenuates myocardial remodeling through inhibition of platelet-derived growth factor and transforming growth factor activation in a rat model of hypertension. Hypertension. 2014;63:1228–34.
Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–6.
Nicin L, Wagner JUG, Luxán G, Dimmeler S. Fibroblast-mediated intercellular crosstalk in the healthy and diseased heart. FEBS Lett. 2022;596:638–54.
Stewart L, Turner NA. Channelling the force to reprogram the matrix: mechanosensitive ion channels in cardiac fibroblasts. Cells. 2021;10:990.
Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87.
Augustin HG, Koh GY. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science. 2017;357:6353.
Anbara T, Sharifi M, Aboutaleb N. Endothelial to mesenchymal transition in the cardiogenesis and cardiovascular diseases. Curr Cardiol Rev. 2020;16:306–14.
Sánchez-Duffhues G. García de Vinuesa A, van de Pol V, Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J Pathol. 2019;247:333–46.
Yan F, Liu X, Ding H, Zhang W. Paracrine mechanisms of endothelial progenitor cells in vascular repair. Acta Histochem. 2022;124: 151833.
Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007;45:A25-32.
Shi J, Yang Y, Cheng A, Xu G, He F. Metabolism of vascular smooth muscle cells in vascular diseases. Am J Physiol Heart Circ Physiol. 2020;319:H613–31.
Mao C, Ma Z, Jia Y, et al. Nidogen-2 maintains the contractile phenotype of vascular smooth muscle cells and prevents neointima formation via bridging jagged1-notch3 signaling. Circulation. 2021;144:1244–61.
Wang L, Zheng J, Du Y, et al. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ Res. 2010;106:514–25.
Cai Z, Xie N, Gong Z, et al. Activin receptor-like kinase 3 directly couples gαq (guanine nucleotide-binding protein subunit αq)/ gαq (guanine nucleotide-binding protein subunit α11) to regulate vascular contractility. Hypertension. 2023;80:1231–44.
Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66.
Kong X, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21:941–54.
Fukui S, Iwamoto N, Takatani A, et al. M1 and M2 monocytes in rheumatoid arthritis: a contribution of imbalance of M1/M2 monocytes to osteoclastogenesis. Front Immunol. 2017;8:1958.
Zhang YH, He M, Wang Y, Liao AH. Modulators of the balance between M1 and M2 macrophages during PREGNANCY. Front Immunol. 2017;8:120.
Fujiu K, Nagai R. Fibroblast-mediated pathways in cardiac hypertrophy. J Mol Cell Cardiol. 2014;70:64–73.
Weber K T, Brilla C G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991 83 1849–65.
Butt RP, Laurent GJ, Bishop JE. Mechanical load and polypeptide growth factors stimulate cardiac fibroblast activity. Ann N Y Acad Sci. 1995;752:387–93.
Montiel-Jaen MG, Monsalvo-Villegas A, Ávila G. Modulating ALDH2 reveals a differential dependence on ROS for hypertrophy and SR Ca(2+) release in aldosterone-treated cardiac myocytes. Biochem Biophys Res Commun. 2021;536:7–13.
Fujisaki H, Ito H, Hirata Y, et al. Natriuretic peptides inhibit angiotensin II-induced proliferation of rat cardiac fibroblasts by blocking endothelin-1 gene expression. J Clin Invest. 1995;96:1059–65.
Calderone A, Thaik CM, Takahashi N, Chang DL, Colucci WS. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest. 1998;101:812–8.
Li P, Wang D, Lucas J, et al. Atrial natriuretic peptide inhibits transforming growth factor beta-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res. 2008;102:185–92.
Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115.
Colliva A, Braga L, Giacca M, Zacchigna S. Endothelial cell-cardiomyocyte crosstalk in heart development and disease. J Physiol. 2020;598:2923–39.
Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC Jr. Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol. 1992;263:H1318–21.
Nakagawa Y, Nishikimi T. CNP, the Third Natriuretic Peptide: Its Biology and Significance to the Cardiovascular System. Biology (Basel). 2022;11:986.
Moyes AJ, Hobbs AJ. C type natriuretic peptide: a multifaceted paracrine regulator in the heart and vasculature. Int J Mol Sci. 2019;20:E2281.
Soeki T, Kishimoto I, Okumura H, et al. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2005;45:608–16.
Špiranec K, Chen W, Werner F, et al. Endothelial C-Type Natriuretic Peptide Acts on Pericytes to Regulate Microcirculatory Flow and Blood Pressure. Circulation. 2018;138:494–508.
Perbellini F, Watson SA, Bardi I, Terracciano CM. Heterocellularity and Cellular Cross-Talk in the Cardiovascular System. Front Cardiovasc Med. 2018;5:143.
Wagner JUG, Dimmeler S. Cellular cross-talks in the diseased and aging heart. J Mol Cell Cardiol. 2020;138:136–46.
Fiebeler A, Schmidt F, Müller DN, et al. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension. 2001;37:787–93.
Caprio M, Newfell BG, la Sala A, et al. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–67.
Tomasoni D, Adamo M, Lombardi CM, Metra M. Highlights in heart failure. ESC Heart Fail. 2019;6:1105–27.
van Empel VP, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res. 2005;67:21–9.
Del Monte F, Hajjar RJ. Intracellular devastation in heart failure. Heart Fail Rev. 2008;13:151–62.
Zuchi C, Tritto I, Carluccio E, Mattei C, Cattadori G, Ambrosio G. Role of endothelial dysfunction in heart failure. Heart Fail Rev. 2020;25:21–30.
Winlaw DS, Smythe GA, Keogh AM, Schyvens CG, Spratt PM, Macdonald PS. Increased nitric oxide production in heart failure. Lancet. 1994;344:373–4.
Belch JJ, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. Br Heart J. 1991;65:245–8.
Bauersachs J, Schäfer A. Endothelial dysfunction in heart failure: mechanisms and therapeutic approaches. Curr Vasc Pharmacol. 2004;2:115–24.
Herum KM, Choppe J, Kumar A, Engler AJ, McCulloch AD. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol Biol Cell. 2017;28:1871–82.
Nagaraju CK, Dries E, Gilbert G, et al. Myofibroblast modulation of cardiac myocyte structure and function. Sci Rep. 2019;9:8879.
Hall C, Gehmlich K, Denning C, Pavlovic D. Complex Relationship Between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease. J Am Heart Assoc. 2021;10: e019338.
Vasquez C, Mohandas P, Louie KL, Benamer N, Bapat AC, Morley GE. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res. 2010;107:1011–20.
Strassheim D, Dempsey EC, Gerasimovskaya E, Stenmark K, Karoor V. Role of Inflammatory Cell Subtypes in Heart Failure. J Immunol Res. 2019;2019:2164017.
Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–29.
Blanda V, Bracale UM, Di Taranto MD, Fortunato G. Galectin-3 in Cardiovascular Diseases. Int J Mol Sci. 2020;21:9232.
Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–6.
Shi X, Jiang X, Chen C, Zhang Y, Sun X. The interconnections between the microtubules and mitochondrial networks in cardiocerebrovascular diseases: Implications for therapy. Pharmacol Res. 2022;184: 106452.
Ashrafian H, McKenna WJ, Watkins H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ Res. 2011;109:86–96.
Shi X, Zhang Y, Gong Y, et al. Zebrafish hhatla is involved in cardiac hypertrophy. J Cell Physiol. 2021;236:3700–9.
Jiang Y, Li X, Guo T, et al. Ranolazine rescues the heart failure phenotype of PLN-deficient human pluripotent stem cell-derived cardiomyocytes. Stem Cell Reports. 2022;17:804–19.
Ho CY, López B, Coelho-Filho OR, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–63.
Meng Q, Bhandary B, Bhuiyan MS, et al. Myofibroblast-specific TGFβ receptor II signaling in the fibrotic response to cardiac myosin binding protein C-Induced cardiomyopathy. Circ Res. 2018;123:1285–97.
Koleini N, Santiago JJ, Nickel BE, et al. Elimination or neutralization of endogenous high-molecular-weight FGF2 mitigates doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2019;316:H279–88.
Bang C, Batkai S, Dangwal S, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–46.
He X, Zeng H, Chen JX. Emerging role of SIRT3 in endothelial metabolism, angiogenesis, and cardiovascular disease. J Cell Physiol. 2019;234:2252–65.
Zhang S, Li Y, Huang X, et al. Seamless genetic recording of transiently activated mesenchymal gene expression in endothelial cells during cardiac fibrosis. Circulation. 2021;144:2004–20.
Peng Q, Shan D, Cui K, et al. The Role of Endothelial-to-Mesenchymal Transition in Cardiovascular Disease. Cells. 2022;11:1834.
Ma F, Li Y, Jia L, et al. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF β/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS ONE. 2012;7: e35144.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–65.
Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119:91–112.
Wang X, Song Q. Mst1 regulates post-infarction cardiac injury through the JNK-Drp1-mitochondrial fission pathway. Cell Mol Biol Lett. 2018;23:21.
Harvey PA, Leinwand LA. The cell biology of disease: cellular mechanisms of cardiomyopathy. J Cell Biol. 2011;194:355–65.
Burke RM, Burgos Villar KN, Small EM. Fibroblast contributions to ischemic cardiac remodeling. Cell Signal. 2021;77: 109824.
Han M, Liu Z, Liu L, et al. Dual genetic tracing reveals a unique fibroblast subpopulation modulating cardiac fibrosis. Nat Genet. 2023;55:665–78.
Croquelois A, Domenighetti AA, Nemir M, et al. Control of the adaptive response of the heart to stress via the Notch1 receptor pathway. J Exp Med. 2008;205:3173–85.
Zhou XL, Fang YH, Wan L, et al. Notch signaling inhibits cardiac fibroblast to myofibroblast transformation by antagonizing TGF-β1/Smad3 signaling. J Cell Physiol. 2019;234:8834–45.
Wattanapitayakul SK, Weinstein DM, Holycross BJ, Bauer JA. Endothelial dysfunction and peroxynitrite formation are early events in angiotensin-induced cardiovascular disorders. Faseb j. 2000;14:271–8.
Zaidi Y, Aguilar EG, Troncoso M, Ilatovskaya DV, DeLeon-Pennell KY. Immune regulation of cardiac fibrosis post myocardial infarction. Cell Signal. 2021;77: 109837.
Lindsey M, Wedin K, Brown MD, et al. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation. 2001;103:2181–7.
Rohde LE, Ducharme A, Arroyo LH, et al. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–70.
Wei Z, Fei Y, Wang Q, et al. Loss of Camk2n1 aggravates cardiac remodeling and malignant ventricular arrhythmia after myocardial infarction in mice via NLRP3 inflammasome activation. Free Radic Biol Med. 2021;167:243–57.
Jiang J, Gu X, Wang H, Ding S. Resveratrol improves cardiac function and left ventricular fibrosis after myocardial infarction in rats by inhibiting NLRP3 inflammasome activity and the TGF-β1/SMAD2 signaling pathway. PeerJ. 2021;9: e11501.
Qiu H, Liu W, Lan T, et al. Salvianolate reduces atrial fibrillation through suppressing atrial interstitial fibrosis by inhibiting TGF-β1/Smad2/3 and TXNIP/NLRP3 inflammasome signaling pathways in post-MI rats. Phytomedicine. 2018;51:255–65.
Kumar R, Sharma A, Pattnaik AK, Varadwaj PK. Stem cells: an overview with respect to cardiovascular and renal disease. J Nat Sci Biol Med. 2010;1:43–52.
Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42.
Ding DC, Shyu WC, Lin SZ, Li H. Current concepts in adult stem cell therapy for stroke. Curr Med Chem. 2006;13:3565–74.
Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11:349.
Lazar E, Benedek T, Korodi S, Rat N, Lo J, Benedek I. Stem cell-derived exosomes - an emerging tool for myocardial regeneration. World J Stem Cells. 2018;10:106–15.
Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14.
Wang Y, Fang J, Liu B, Shao C, Shi Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell. 2022;29:1515–30.
Gao L, Qiu F, Cao H, et al. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics. 2023;13:685–703.
Mokhtari B, Aboutaleb N, Nazarinia D, et al. Comparison of the effects of intramyocardial and intravenous injections of human mesenchymal stem cells on cardiac regeneration after heart failure. Iran J Basic Med Sci. 2020;23:879–85.
Piao H, Youn TJ, Kwon JS, et al. Effects of bone marrow derived mesenchymal stem cells transplantation in acutely infarcting myocardium. Eur J Heart Fail. 2005;7:730–8.
Kawamoto A, Asahara T, Losordo DW. Transplantation of endothelial progenitor cells for therapeutic neovascularization. Cardiovasc Radiat Med. 2002;3:221–5.
Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–7.
Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–79.
He J, Teng X, Yu Y, et al. Injection of Sca-1+/CD45+/CD31+ mouse bone mesenchymal stromal-like cells improves cardiac function in a mouse myocardial infarct model. Differentiation. 2013;86:57–64.
Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197:496–503.
Murayama T, Tepper OM, Silver M, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002;30:967–72.
Di Santo S, Yang Z, Wyler von Ballmoos M, et al. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE. 2009;4: e5643.
Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8.
Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7.
Stastna M, Abraham MR, Van Eyk JE. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett. 2009;583:1800–7.
Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–93.
Wang QR, Wang BH, Zhu WB, Huang YH, Li Y, Yan Q. An in vitro study of differentiation of hematopoietic cells to endothelial cells. Bone Marrow Res. 2011;2011: 846096.
Prunier F, Pfister O, Hadri L, et al. Delayed erythropoietin therapy reduces post-MI cardiac remodeling only at a dose that mobilizes endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2007;292:H522–9.
Döbert N, Britten M, Assmus B, et al. Transplantation of progenitor cells after reperfused acute myocardial infarction: evaluation of perfusion and myocardial viability with FDG-PET and thallium SPECT. Eur J Nucl Med Mol Imaging. 2004;31:1146–51.
Olivieri F, Mazzanti I, Abbatecola AM, et al. Telomere/Telomerase system: a new target of statins pleiotropic effect? Curr Vasc Pharmacol. 2012;10:216–24.
Lee PS, Poh KK. Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells. 2014;6:355–66.
Ebrahimi M, Forouzesh M, Raoufi S, Ramazii M, Ghaedrahmati F, Farzaneh M. Differentiation of human induced pluripotent stem cells into erythroid cells. Stem Cell Res Ther. 2020;11:483.
Fan D, Wu H, Pan K, Peng H, Wu R. Regenerating Damaged Myocardium: A Review of Stem-Cell Therapies for Heart Failure. Cells. 2021;10:3125.
Menasché P. Embryonic stem cells for severe heart failure: why and how? J Cardiovasc Transl Res. 2012;5:555–65.
Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–9.
Maliken BD, Molkentin JD. Undeniable Evidence That the Adult Mammalian Heart Lacks an Endogenous Regenerative Stem Cell. Circulation. 2018;138:806–8.
Zhang J, Tian X, Peng C, et al. Transplantation of CREG modified embryonic stem cells improves cardiac function after myocardial infarction in mice. Biochem Biophys Res Commun. 2018;503:482–9.
Catelain C, Riveron S, Papadopoulos A, et al. Myoblasts and embryonic stem cells differentially engraft in a mouse model of genetic dilated cardiomyopathy. Mol Ther. 2013;21:1064–75.
Oettgen P, Boyle AJ, Schulman SP, et al. Cardiac stem cell therapy need for optimization of efficacy and safety monitoring. Circulation. 2006;114(353):8.
Li Z, Wilson KD, Smith B, et al. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS ONE. 2009;4: e8443.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Karagiannis P, Takahashi K, Saito M, et al. Induced pluripotent stem cells and their use in human models of disease and development. Physiol Rev. 2019;99:79–114.
Rostovskaya M, Bredenkamp N, Smith A. Towards consistent generation of pancreatic lineage progenitors from human pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140365.
Drakhlis L, Biswanath S, Farr CM, et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat Biotechnol. 2021;39:737–46.
Oksanen M, Petersen AJ, Naumenko N, et al. PSEN1 mutant iPSC-Derived model reveals severe astrocyte pathology in Alzheimer’s disease. Stem Cell Reports. 2017;9:1885–97.
Kashiyama N, Miyagawa S, Fukushima S, et al. MHC-mismatched allotransplantation of induced pluripotent stem cell-derived cardiomyocyte sheets to improve cardiac function in a primate ischemic cardiomyopathy model. Transplantation. 2019;103:1582–90.
Zhao M, Nakada Y, Wei Y, et al. Cyclin D2 overexpression enhances the efficacy of human induced pluripotent stem cell-derived cardiomyocytes for myocardial repair in a swine model of myocardial infarction. Circulation. 2021;144:210–28.
Mallapaty S. Revealed: two men in China were first to receive pioneering stem-cell treatment for heart disease. Nature. 2020;581:249–50.
Kawamura T, Ito Y, Ito E, et al. Safety confirmation of induced pluripotent stem cell-derived cardiomyocyte patch transplantation for ischemic cardiomyopathy: first three case reports. Front Cardiovasc Med. 2023;10:1182209.
Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21:145–61.
Amini L, Silbert SK, Maude SL, et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat Rev Clin Oncol. 2022;19:342–55.
Rurik JG, Tombácz I, Yadegari A, et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91–6.
Hampton T. Exploring the Potential of CAR-T Therapy for Heart Failure. JAMA. 2019;322:2066–7.
Dalal PJ, Patel NP, Feinstein MJ, Akhter N. Adverse cardiac effects of car t-cell therapy: characteristics, surveillance, management, and future research directions. Technol Cancer Res Treat. 2022;21:15330338221132928.
Burstein DS, Maude S, Grupp S, Griffis H, Rossano J, Lin K. Cardiac profile of chimeric antigen receptor T cell therapy in children: a single-institution experience. Biol Blood Marrow Transplant. 2018;24:1590–5.
Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR, Scherrer-Crosbie M. Cardiovascular effects of CAR T cell therapy: a retrospective study. JACC CardioOncol. 2020;2:193–203.
Ganatra S, Redd R, Hayek SS, et al. Chimeric antigen receptor T-Cell therapy-associated cardiomyopathy in patients with refractory or relapsed non-hodgkin lymphoma. Circulation. 2020;142:1687–90.
Zhang JP, Li XL, Li GH, et al. Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol. 2017;18:35.
Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23:R40–6.
Xiao-Jie L, Hui-Ying X, Zun-Ping K, Jin-Lian C, Li-Juan J. CRISPR-Cas9: a new and promising player in gene therapy. J Med Genet. 2015;52:289–96.
Xie C, Zhang YP, Song L, et al. Genome editing with CRISPR/Cas9 in postnatal mice corrects PRKAG2 cardiac syndrome. Cell Res. 2016;26:1099–111.
Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol. 2012;60:705–15.
Ma H, Marti-Gutierrez N, Park SW, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548:413–9.
Ran FA, Cong L, Yan WX, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–91.
Ding Q, Strong A, Patel KM, et al. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ Res. 2014;115:488–92.
Kaneko M, Hashikami K, Yamamoto S, Matsumoto H, Nishimoto T. Phospholamban Ablation Using CRISPR/Cas9 System Improves Mortality in a Murine Heart Failure Model. PLoS ONE. 2016;11: e0168486.
Ma Y, Zhang L, Huang X. Genome modification by CRISPR/Cas9. Febs j. 2014;281:5186–93.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82170370, 81770381), the Natural Science Foundation of Jiangsu Province (BK20211165) and Zhishan Youth Scholar Program of SEU.
Author information
Authors and Affiliations
Contributions
Jiayu Yao: prepared the original draft. Yuejun Chen: designed and prepared the figures. Yuqing Huang: collected the literature. Xiaoou Sun: manuscript revision. Xingjuan Shi: manuscript design, supervision and revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
Not applicable.
Informed consent Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, J., Chen, Y., Huang, Y. et al. The role of cardiac microenvironment in cardiovascular diseases: implications for therapy. Human Cell 37, 607–624 (2024). https://doi.org/10.1007/s13577-024-01052-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-024-01052-3