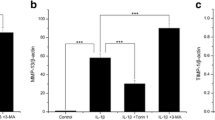
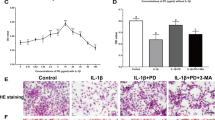
Abstract
Osteoarthritis (OA) is the main pathogenic factor in diseases that cause joint deformities. As the main manifestation of the progress of OA, cartilage degradation has been closely associated with the degeneration of chondrocytes, which is induced by inflammatory factors and other trauma factors. Autophagy and apoptosis are the main mechanisms for cells to maintain homeostasis and play crucial roles in OA. Under the influence of external environmental factors (such as aging and injury), the metabolism of cells can be altered, which may affect the extent of autophagy and apoptosis. With the progression of OA, these changes can alter the cell phenotypes, and the cells of different phenotypes display distinct differences in morphology and function. In this review, we have summarized the alteration in cell metabolism, autophagy, and the extent of apoptosis during OA progression and its effects on the cell phenotypes to provide new ideas for further research on the mechanisms of phenotypic transition and therapeutic strategies so as to reverse the cell phenotypes.



Similar content being viewed by others
Availability of data and materials (ADM)
The authors confirmed that the data supporting the findings of this study are available within the article. Any requests for data can be sent to Prof. Mao Nie (Email: 302218@cqmu.edu.cn).
References
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet (London, England). 2019;393:1745–59.
Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis. 2010;15:631–8.
Zheng L, Zhang Z, Sheng P, Mobasheri A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev. 2021;66: 101249.
Jeon OH, David N, Campisi J, Elisseeff JH. Senescent cells and osteoarthritis: a painful connection. J Clin Invest. 2018;128:1229–37.
Coryell PR, Diekman BO, Loeser RF. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat Rev Rheumatol. 2021;17:47–57.
Duan R, Xie H, Liu ZZ. The role of autophagy in osteoarthritis. Front Cell Develop Biol. 2020;8: 608388.
Li YS, Zhang FJ, Zeng C, et al. Autophagy in osteoarthritis Joint bone spine. 2016;83:143–8.
Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11:35–44.
Wang C, Yao Z, Zhang Y, et al. Metformin Mitigates Cartilage Degradation by Activating AMPK/SIRT1-Mediated Autophagy in a Mouse Osteoarthritis Model. Front Pharmacol. 2020;11:1114.
Lin Z, Miao J, Zhang T, et al. JUNB-FBXO21-ERK axis promotes cartilage degeneration in osteoarthritis by inhibiting autophagy. Aging Cell. 2021;20: e13306.
Wang S, Deng Z, Ma Y, et al. The Role of Autophagy and Mitophagy in Bone Metabolic Disorders. Int J Biol Sci. 2020;16:2675–91.
Mei R, Lou P, You G, Jiang T, Yu X, Guo L. 17β-Estradiol Induces Mitophagy Upregulation to Protect Chondrocytes via the SIRT1-Mediated AMPK/mTOR Signaling Pathway. Front Endocrinol. 2020;11: 615250.
Chen Y, Wu YY, Si HB, Lu YR, Shen B. Mechanistic insights into AMPK-SIRT3 positive feedback loop-mediated chondrocyte mitochondrial quality control in osteoarthritis pathogenesis. Pharmacol Res. 2021;166: 105497.
Zhou J, Wang Y, Liu Y, Zeng H, Xu H, Lian F. Adipose derived mesenchymal stem cells alleviated osteoarthritis and chondrocyte apoptosis through autophagy inducing. Journal of cellular biochemistry. 2018.
D’Adamo S, Cetrullo S, Minguzzi M, Silvestri Y, Borzì RM, Flamigni F. MicroRNAs and Autophagy: Fine Players in the Control of Chondrocyte Homeostatic Activities in Osteoarthritis. Oxid Med Cell Longev. 2017;2017:3720128.
Wu J, Kuang L, Chen C, et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100.
Tang Q, Zheng G, Feng Z, et al. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis. 2017;8: e3081.
Hosseinzadeh A, Kamrava SK, Joghataei MT, et al. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J Pineal Res. 2016;61:411–25.
Park DR, Kim J, Kim GM, et al. Osteoclast-associated receptor blockade prevents articular cartilage destruction via chondrocyte apoptosis regulation. Nat Commun. 2020;11:4343.
Guo Q, Chen X, Chen J, et al. STING promotes senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the NF-κB signaling pathway. Cell Death Dis. 2021;12:13.
Wang B, Shao Z, Gu M, et al. Hydrogen sulfide protects against IL-1β-induced inflammation and mitochondrial dysfunction-related apoptosis in chondrocytes and ameliorates osteoarthritis. J Cell Physiol. 2021;236:4369–86.
Xu K, He Y, Moqbel SAA, Zhou X, Wu L, Bao J. SIRT3 ameliorates osteoarthritis via regulating chondrocyte autophagy and apoptosis through the PI3K/Akt/mTOR pathway. Int J Biol Macromol. 2021;175:351–60.
Li K, Yang P, Zhang Y, et al. DEPTOR Prevents Osteoarthritis Development Via Interplay With TRC8 to Reduce Endoplasmic Reticulum Stress in Chondrocytes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2021;36:400–11.
Cao Y, Tang S, Nie X, et al. Decreased miR-214-3p activates NF-κB pathway and aggravates osteoarthritis progression. EBioMedicine. 2021;65: 103283.
Xiaoshi J, Maoquan L, Jiwei W, Jinqiu N, Ke Z. SETD7 mediates the vascular invasion in articular cartilage and chondrocytes apoptosis in osteoarthriis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2021;35: e21283.
Zhang P, Liu Y, Jia L, et al. SP600125, a JNK-Specific Inhibitor, Regulates in vitro Auricular Cartilage Regeneration by Promoting Cell Proliferation and Inhibiting Extracellular Matrix Metabolism. Frontiers in cell and developmental biology. 2021;9: 630678.
He Y, Fan L, Aaron N, et al. Reduction of Smad2 caused by oxidative stress leads to necrotic death of hypertrophic chondrocytes associated with an endemic osteoarthritis. Rheumatology (Oxford, England). 2021.
Jiang S, Liu Y, Xu B, Zhang Y, Yang M. Noncoding RNAs: New regulatory code in chondrocyte apoptosis and autophagy. Wiley interdisciplinary reviews RNA. 2020;11: e1584.
Rosa SC, Gonçalves J, Judas F, Mobasheri A, Lopes C, Mendes AF. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res Ther. 2009;11:R80.
Wang C, Silverman RM, Shen J, O’Keefe RJ. Distinct metabolic programs induced by TGF-β1 and BMP2 in human articular chondrocytes with osteoarthritis. Journal of orthopaedic translation. 2018;12:66–73.
Li ZZ, Wang F, Liu S, Li H, Wang Y. Ablation of PKM2 ameliorated ER stress-induced apoptosis and associated inflammation response in IL-1β-treated chondrocytes via blocking Rspo2-mediated Wnt/β-catenin signaling. J Cell Biochem. 2020;121:4204–13.
Song J, Baek IJ, Chun CH, Jin EJ. Dysregulation of the NUDT7-PGAM1 axis is responsible for chondrocyte death during osteoarthritis pathogenesis. Nat Commun. 2018;9:3427.
Nogueira-Recalde U, Lorenzo-Gómez I, Blanco FJ, et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine. 2019;45:588–605.
Rocha B, Cillero-Pastor B, Ruiz-Romero C, et al. Identification of a distinct lipidomic profile in the osteoarthritic synovial membrane by mass spectrometry imaging. Osteoarthritis and cartilage. 2021.
Huang MJ, Wang L, Jin DD, et al. Enhancement of the synthesis of n-3 PUFAs in fat-1 transgenic mice inhibits mTORC1 signalling and delays surgically induced osteoarthritis in comparison with wild-type mice. Ann Rheum Dis. 2014;73:1719–27.
Choi WS, Lee G, Song WH, et al. The CH25H-CYP7B1-RORα axis of cholesterol metabolism regulates osteoarthritis. Nature. 2019;566:254–8.
Farnaghi S, Prasadam I, Cai G, et al. Protective effects of mitochondria-targeted antioxidants and statins on cholesterol-induced osteoarthritis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2017;31:356–67.
Wei Y, Yan L, Luo L, et al. Phospholipase A(2) inhibitor-loaded micellar nanoparticles attenuate inflammation and mitigate osteoarthritis progression. Science advances. 2021; 7.
Ma CH, Chiua YC, Wu CH, et al. Homocysteine causes dysfunction of chondrocytes and oxidative stress through repression of SIRT1/AMPK pathway: A possible link between hyperhomocysteinemia and osteoarthritis. Redox Biol. 2018;15:504–12.
Riegger J, Joos H, Palm HG, et al. Antioxidative therapy in an ex vivo human cartilage trauma-model: attenuation of trauma-induced cell loss and ECM-destructive enzymes by N-acetyl cysteine. Osteoarthritis Cartilage. 2016;24:2171–80.
Ji ML, Jiang H, Wu F, et al. Precise targeting of miR-141/200c cluster in chondrocytes attenuates osteoarthritis development. Annals of the rheumatic diseases. 2020.
Loeser RF, Kelley KL, Armstrong A, Collins JA, Diekman BO, Carlson CS. Deletion of JNK Enhances Senescence in Joint Tissues and Increases the Severity of Age-Related Osteoarthritis in Mice. Arthritis & rheumatology (Hoboken, NJ). 2020;72:1679–88.
Zhang Y, Pizzute T, Li J, He F, Pei M. sb203580 preconditioning recharges matrix-expanded human adult stem cells for chondrogenesis in an inflammatory environment - A feasible approach for autologous stem cell based osteoarthritic cartilage repair. Biomaterials. 2015;64:88–97.
Zhao X, Wang T, Cai B, et al. MicroRNA-495 enhances chondrocyte apoptosis, senescence and promotes the progression of osteoarthritis by targeting AKT1. American journal of translational research. 2019;11:2232–44.
Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412–20.
Zheng G, Zhan Y, Li X, et al. TFEB, a potential therapeutic target for osteoarthritis via autophagy regulation. Cell Death Dis. 2018;9:858.
Portal-Núñez S, Esbrit P, Alcaraz MJ, Largo R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem Pharmacol. 2016;108:1–10.
Zhao X, Huang P, Li G, Lv Z, Hu G, Xu Q. Activation of the leptin pathway by high expression of the long form of the leptin receptor (Ob-Rb) accelerates chondrocyte senescence in osteoarthritis. Bone & joint research. 2019;8:425–36.
Singh P, Marcu KB, Goldring MB, Otero M. Phenotypic instability of chondrocytes in osteoarthritis: on a path to hypertrophy. Ann N Y Acad Sci. 2019;1442:17–34.
Tong W, Zeng Y, Chow DHK, et al. Wnt16 attenuates osteoarthritis progression through a PCP/JNK-mTORC1-PTHrP cascade. Ann Rheum Dis. 2019;78:551–61.
Kang X, Qian Z, Liu J, et al. Neuropeptide Y Acts Directly on Cartilage Homeostasis and Exacerbates Progression of Osteoarthritis Through NPY2R. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2020;35:1375–84.
Bertrand J, Kräft T, Gronau T, et al. BCP crystals promote chondrocyte hypertrophic differentiation in OA cartilage by sequestering Wnt3a. Ann Rheum Dis. 2020;79:975–84.
Lin NY, Distler A, Beyer C, et al. Inhibition of Notch1 promotes hedgehog signalling in a HES1-dependent manner in chondrocytes and exacerbates experimental osteoarthritis. Ann Rheum Dis. 2016;75:2037–44.
Wu D, Jin S, Lin Z, et al. Sauchinone inhibits IL-1β induced catabolism and hypertrophy in mouse chondrocytes to attenuate osteoarthritis via Nrf2/HO-1 and NF-κB pathways. Int Immunopharmacol. 2018;62:181–90.
Markway BD, Cho H, Johnstone B. Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis Res Ther. 2013;15:R92.
Yano F, Ohba S, Murahashi Y, Tanaka S, Saito T, Chung UI. Runx1 contributes to articular cartilage maintenance by enhancement of cartilage matrix production and suppression of hypertrophic differentiation. Sci Rep. 2019;9:7666.
Chou HC, Chen CH, Chou LY, et al. Discoidin Domain Receptors 1 Inhibition Alleviates Osteoarthritis via Enhancing Autophagy. International journal of molecular sciences. 2020; 21.
Yue J, Jin S, Gu S, Sun R, Liang Q. High concentration magnesium inhibits extracellular matrix calcification and protects articular cartilage via Erk/autophagy pathway. J Cell Physiol. 2019;234:23190–201.
Rim YA, Nam Y, Ju JH. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. International journal of molecular sciences. 2020; 21.
Wei F, Zhou J, Wei X, et al. Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2012;20:755–63.
Varela-Eirin M, Loureiro J, Fonseca E, et al. Cartilage regeneration and ageing: Targeting cellular plasticity in osteoarthritis. Ageing Res Rev. 2018;42:56–71.
Niada S, Giannasi C, Gomarasca M, Stanco D, Casati S, Brini AT. Adipose-derived stromal cell secretome reduces TNFα-induced hypertrophy and catabolic markers in primary human articular chondrocytes. Stem cell research. 2019;38: 101463.
Huang X, You Y, Xi Y, et al. p-Coumaric Acid Attenuates IL-1β-Induced Inflammatory Responses and Cellular Senescence in Rat Chondrocytes. Inflammation. 2020;43:619–28.
López-Alcorocho JM, Guillén-Vicente I, Rodríguez-Iñigo E, et al. Study of Telomere Length in Preimplanted Cultured Chondrocytes. Cartilage. 2019;10:36–42.
Almeida M, Porter RM. Sirtuins and FoxOs in osteoporosis and osteoarthritis. Bone. 2019;121:284–92.
Duarte JH. Osteoarthritis: SIRT6 prevents chondrocyte senescence and DNA damage. Nat Rev Rheumatol. 2015;11:260.
Clérigues V, Guillén MI, Gomar F, Alcaraz MJ. Haem oxygenase-1 counteracts the effects of interleukin-1β on inflammatory and senescence markers in cartilage-subchondral bone explants from osteoarthritic patients. Clinical science (London, England : 1979). 2012; 122:239–50.
Goutas A, Papathanasiou I, Mourmoura E, Tsesmelis K, Tsezou A, Trachana V. Oxidative Stress Response Is Mediated by Overexpression and Spatiotemporal Regulation of Caveolin-1. Antioxidants (Basel, Switzerland). 2020; 9.
Au M, Liu Z, Rong L, Zheng Y, Wen C. Endothelin-1 induces chondrocyte senescence and cartilage damage via endothelin receptor type B in a post-traumatic osteoarthritis mouse model. Osteoarthritis Cartilage. 2020;28:1559–71.
Jeon OH, Kim C, Laberge RM, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–81.
Kuwahara M, Kadoya K, Kondo S, et al. CCN3 (NOV) Drives Degradative Changes in Aging Articular Cartilage. International journal of molecular sciences. 2020; 21.
Ji Q, Zheng Y, Zhang G, et al. Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. Ann Rheum Dis. 2019;78:100–10.
Chen P, Xia C, Mei S, et al. Intra-articular delivery of sinomenium encapsulated by chitosan microspheres and photo-crosslinked GelMA hydrogel ameliorates osteoarthritis by effectively regulating autophagy. Biomaterials. 2016;81:1–13.
Peilin W, Songsong T, Chengyu Z, et al. Directed elimination of senescent cells attenuates development of osteoarthritis by inhibition of c-IAP and XIAP. Biochim Biophys Acta. 2019;1865:2618–32.
Hwang HS, Kim HA. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int J Mol Sci. 2015;16:26035–54.
Wang Y, Xu J, Zhang X, et al. TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis. 2017;8: e2715.
Wang YH, Kuo SJ, Liu SC, et al. Apelin Affects the Progression of Osteoarthritis by Regulating VEGF-Dependent Angiogenesis and miR-150–5p Expression in Human Synovial Fibroblasts. Cells. 2020; 9.
Zhang H, Lin C, Zeng C, et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis. 2018;77:1524–34.
Dai M, Sui B, Xue Y, Liu X, Sun J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials. 2018;180:91–103.
Acknowledgements
Mao Nie was supported by the Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University.
Funding
This paper was supported by grants from the Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau, 2023MSXM141) and the Natural Science Foundation of Chongqing [No. cstc2020jcyj-msxmX0806, Ting Wang].
Author information
Authors and Affiliations
Contributions
ZL: writing—original draft, review, and editing. XS: methodology and writing—review and editing. TW: funding support. MN: funding support and writing—review and editing. All authors read and approved the manuscript. Xianding Sun and Mao Nie contributed equally.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declared that they have no conflicts of interest to regarding this work. We declare that we do not have any commercial or associative interest conflicts in connection with the work submitted.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Wang, T., Sun, X. et al. Autophagy and apoptosis: regulatory factors of chondrocyte phenotype transition in osteoarthritis. Human Cell 36, 1326–1335 (2023). https://doi.org/10.1007/s13577-023-00926-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00926-2