Abstract
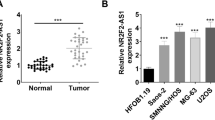
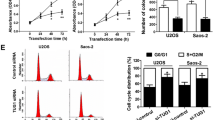
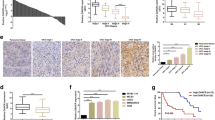
Osteosarcoma (OSA), the malignant bone tumor, predominantly affecting children and adolescents, threatens the life and life quality of the patients. An increasing number of studies have indicated the role of long non-coding RNA (lncRNA) dysregulation in cancer biology. Herein, the study was aimed to explore the role of FGD5 antisense RNA 1 (FGD5-AS1), a lncRNA, in OSA. Expression levels of FGD5-AS1, miR-506-3p and RAB3D mRNA were quantified utilizing qRT-PCR. The expression of RAB3D protein was examined employing Western blot. A series of functional experiments including CCK-8 assay, BrdU assay, wound healing assay, Transwell assay were performed for studying the effects of FGD5-AS1 on the malignancy of OSA cell lines 143B and HOS. The binding site between miR-506-3p and FGD5-AS1 was identified and validated by luciferase reporter assay and RNA immunoprecipitation assay. It was demonstrated that the expression of FGD5-AS1 was up-regulated in OSA tissues and cell lines, and its high expression is associated with higher Enneking stage and poorer histological differentiation. Gain-of-function and loss-of-function studies suggested that FGD5-AS1 facilitated OSA cells proliferation and migration. The promoting effects of FGD5-AS1 overexpression on OSA cell proliferation and migration could be counteracted by miR-506-3p. Moreover, FGD5-AS1 competitively adsorbed miR-506-3p to repress its expression so as to up-regulate the expression of RAB3D. These results indicate that FGD5-AS1 is capable of expediting OSA cell proliferation and migration via sponging miR-506-3p to up-regulate RAB3D.







Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017;13:357–68.
Liu YJ, Li W, Chang F, et al. MicroRNA-505 is downregulated in human osteosarcoma and regulates cell proliferation, migration and invasion. Oncol Rep. 2018;39:491–500.
Simpson S, Dunning MD, de Brot S, et al. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand. 2017;59:71.
Wang J, Su Z, Lu S, et al. LncRNA HOXA-AS2 and its molecular mechanisms in human cancer. ClinChimActa. 2018;485:229–33.
Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965–81.
Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–7.
Wei GH, Wang X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur Rev Med PharmacolSci. 2017;21:3850–6.
Liu Y, Yang Y, Li L, et al. LncRNA SNHG1 enhances cell proliferation, migration, and invasion in cervical cancer. Biochem Cell Biol. 2018;96:38–43.
Ren D, Zheng H, Fei S, et al. MALAT1 induces osteosarcoma progression by targeting miR-206/CDK9 axis. J Cell Physiol. 2018;234:950–7.
Zheng S, Jiang F, Ge D, et al. LncRNA SNHG3/miRNA-151a-3p/RAB22A axis regulates invasion and migration of osteosarcoma. Biomed Pharmacother. 2019;112:108695.
Zhang Y, Meng W, Cui H. LncRNA CBR3-AS1 predicts unfavorable prognosis and promotes tumorigenesis in osteosarcoma. Biomed Pharmacother. 2018;102:169–74.
Jiang R, Tang J, Chen Y, et al. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun. 2017;8:15129.
Liu XH, Sun M, Nie FQ, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92.
Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407.
Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206.
Sanchez Calle A, Kawamura Y, Yamamoto Y, et al. Emerging roles of long non-coding RNA in cancer. CancerSci. 2018;109:2093–100.
Loewen G, Jayawickramarajah J, Zhuo Y, et al. Functions of lncRNA HOTAIR in lung cancer. J HematolOncol. 2014;7:90.
Kumar MM, Goyal R. LncRNA as a therapeutic target for angiogenesis. Curr Top Med Chem. 2017;17:1750–7.
Yang Z, Li X, Yang Y, et al. Long noncoding RNAs in the progression, metastasis, and prognosis of osteosarcoma. Cell Death Dis. 2016;7:e2389.
Yao H, Hou G, Wang QY, et al. LncRNA SPRY4-IT1 promotes progression of osteosarcoma by regulating ZEB1 and ZEB2 expression through sponging of miR-101 activity. Int J Oncol. 2020;56:85–100.
Duan G, Zhang C, Xu C, et al. Knockdown of MALAT1 inhibits osteosarcoma progression via regulating the miR-34a/cyclin D1 axis. Int J Oncol. 2019;54:17–28.
Yin R, Liu J, Zhao D, et al. Long non-coding RNA ASB16-AS1 functions as a miR-760 sponge to facilitate the malignant phenotype of osteosarcoma by increasing HDGF expression. Onco Targets Ther. 2020;13:2261–74.
Yan L, Wu X, Yin X, et al. LncRNA CCAT2 promoted osteosarcoma cell proliferation and invasion. J Cell Mol Med. 2018;22:2592–9.
Li D, Jiang X, Zhang X, et al. Long noncoding RNA FGD5-AS1 promotes colorectal cancer cell proliferation, migration, and invasion through upregulating CDCA7 via sponging miR-302e. Vitro Cell Dev BiolAnim. 2019;55:577–85.
Fan Y, Li H, Yu Z, et al. Long non-coding RNA FGD5-AS1 promotes non-small cell lung cancer cell proliferation through sponging hsa-miR-107 to up-regulate FGFRL1. 2020. Biosci Rep. https://doi.org/10.1042/BSR20193309.
Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314.
Chen G, Zhou H. MiRNA-708/CUL4B axis contributes into cell proliferation and apoptosis of osteosarcoma. Eur Rev Med PharmacolSci. 2018;22:5452–9.
Yuan G, Zhao Y, Wu D, et al. miRNA-20a upregulates TAK1 and increases proliferation in osteosarcoma cells. Future Oncol. 2018;14:461–9.
Hu CY, You P, Zhang J, et al. MiR-506-3p acts as a novel tumor suppressor in prostate cancer through targeting GALNT4. Eur Rev Med PharmacolSci. 2019;23:5133–8.
Wu L, Chen Z, Xing Y. MiR-506–3p inhibits cell proliferation, induces cell cycle arrest and apoptosis in retinoblastoma by directly targeting NEK6. Cell BiolInt. 2018. https://doi.org/10.1002/cbin.11041.
Jiashi W, Chuang Q, Zhenjun Z, et al. MicroRNA-506-3p inhibits osteosarcoma cell proliferation and metastasis by suppressing RAB3D expression. Aging. 2018;10:1294–305.
Wang D, Bao F, Teng Y, et al. MicroRNA-506-3p initiates mesenchymal-to-epithelial transition and suppresses autophagy in osteosarcoma cells by directly targeting SPHK1. BiosciBiotechnolBiochem. 2019;83:836–44.
Qi X, Zhang DH, Wu N, et al. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015;52:710–8.
Wang Y, Zeng X, Wang N, et al. Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1-mediated proliferation and metastasis via decoying of miR-335-5p and miR-1972 in osteosarcoma. Mol Cancer. 2018;17:89.
Cao J, Han X, Qi X, et al. TUG1 promotes osteosarcoma tumorigenesis by upregulating EZH2 expression via miR-144-3p. Int J Oncol. 2017;51:1115–23.
Han N, Zuo L, Chen H, et al. Long non-coding RNA homeobox A11 antisense RNA (HOXA11-AS) promotes retinoblastoma progression via sponging miR-506-3p. Onco Targets Ther. 2019;12:3509–17.
Zhang J, Kong R, Sun L. Silencing of Rab3D suppresses the proliferation and invasion of esophageal squamous cell carcinoma cells. Biomed Pharmacother. 2017;91:402–7.
Millar AL, Pavios NJ, Xu J, et al. Rab3D: a regulator of exocytosis in non-neuronal cells. HistolHistopathol. 2002;17:929–36.
Luo Y, Ye GY, Qin SL, et al. High expression of Rab3D predicts poor prognosis and associates with tumor progression in colorectal cancer. Int J Biochem Cell Biol. 2016;75:53–62.
Cao K, Fang Y, Wang H, et al. ThelncRNA HOXA11-AS regulates Rab3D expression by sponging miR-125a-5p promoting metastasis of osteosarcoma. Cancer Manag Res. 2019;11:4505–18.
Funding
This study was supported by Scientific research support fund for Teachers of Jining Medical College. Project Name: Study on the correlation between mir-202-5p site, XIAP and other related proteins in osteosarcoma (ID: JYFC2018FKJ034).
Author information
Authors and Affiliations
Contributions
BW and CL designed the study. CL and XL conducted most of the experiments and wrote the manuscript. CZ, LW, and JY conducted the experiments and analyzed the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Our study was approved by the Ethics Review Board of People's Hospital of Rizhao (ID: 201703036).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, C., Lin, X., Zhang, C. et al. Long non-coding RNA FGD5-AS1 enhances osteosarcoma cell proliferation and migration by targeting miR-506-3p/RAB3D axis. Human Cell 34, 1255–1265 (2021). https://doi.org/10.1007/s13577-021-00536-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00536-w