Abstract
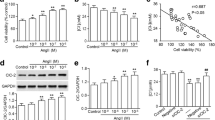
Cerebrovascular smooth muscle cell proliferation is the major contributor to cerebrovascular remodeling and stroke. Chloride channels have been suggested to play an important role in the regulation of smooth muscle cell proliferation. This study aims to investigate the effect of leucine-rich repeat-containing 8A (LRRC8A), an essential component of volume-sensitive chloride channels, on cerebrovascular smooth muscle cell proliferation. The data showed that LRRC8A expression was increased in mouse brain artery during angiotensin II (AngII)-induced cerebrovascular remodeling. Similarly, AngII also increased the expression of LRRC8A in human brain vascular smooth muscle cells (HBVSMCs). Knockdown of LRRC8A by siRNA significantly inhibited AngII-induced the proliferation, migration, and invasion in HBVSMCs. The inhibition of HBVSMCs proliferation by LRRC8A downregulation appeared to be involved in suppression of cell-cycle transition. AngII-induced the decrease in p21 and p27 expression and the increase in CDK4 and cyclin D1 expression were attenuated by LRRC8A downregulation. Moreover, inhibition of LRRC8A suppressed AngII-induced PI3K/AKT activation and reactive oxygen species generation, but had no effect on JNK, ERK, and p38 phosphorylation. In addition, activation of PI3K/AKT-signaling pathways with specific agonists significantly abolished the effect of LRRC8A deficiency on HBVSMC proliferation. This present study demonstrates that knockdown of LRRC8A ameliorates AngII-induced cerebrovascular smooth muscle cell proliferation via inhibiting PI3K/AKT pathway, suggesting that LRRC8A may be a novel molecular target in the treatment of vascular remodeling and stroke.





Similar content being viewed by others
References
Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–71. https://doi.org/10.1161/CIRCULATIONAHA.116.025250.
Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 Study. Neuroepidemiology. 2015;45(3):161–76. https://doi.org/10.1159/000441085.
Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42(12):3651–4. https://doi.org/10.1161/STROKEAHA.111.635755.
Mancia G, Messerli F, Bakris G, Zhou Q, Champion A, Pepine CJ. Blood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril Study. Hypertension. 2007;50(2):299–305. https://doi.org/10.1161/HYPERTENSIONAHA.107.090290.
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–209. https://doi.org/10.1161/CIR.0b013e3182009701.
Galatioto J, Caescu CI, Hansen J, Cook JR, Miramontes I, Iyengar R, et al. Cell type-specific contributions of the angiotensin II type 1a receptor to aorta homeostasis and aneurysmal disease-brief report. Arterioscler Thromb Vasc Biol. 2018;38(3):588–91. https://doi.org/10.1161/ATVBAHA.117.310609.
Ma MM, Lin CX, Liu CZ, Gao M, Sun L, Tang YB, et al. Threonine532 phosphorylation in ClC-3 channels is required for angiotensin II-induced Cl(−) current and migration in cultured vascular smooth muscle cells. Br J Pharmacol. 2016;173(3):529–44. https://doi.org/10.1111/bph.13385.
Yaghini FA, Song CY, Lavrentyev EN, Ghafoor HU, Fang XR, Estes AM, et al. Angiotensin II-induced vascular smooth muscle cell migration and growth are mediated by cytochrome P450 1B1-dependent superoxide generation. Hypertension. 2010;55(6):1461–7. https://doi.org/10.1161/HYPERTENSIONAHA.110.150029.
Chan SH, Chan JY. Angiotensin-generated reactive oxygen species in brain and pathogenesis of cardiovascular diseases. Antioxid Redox Signal. 2013;19(10):1074–84. https://doi.org/10.1089/ars.2012.4585.
Wang XM, Xiao H, Liu LL, Cheng D, Li XJ, Si LY. FGF21 represses cerebrovascular aging via improving mitochondrial biogenesis and inhibiting p53 signaling pathway in an AMPK-dependent manner. Exp Cell Res. 2016;346(2):147–56. https://doi.org/10.1016/j.yexcr.2016.06.020.
Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177(2):119–47. https://doi.org/10.1046/j.1365-201X.2003.01060.x.
Lu J, Xu F, Zhang Y, Lu H, Zhang J. ClC-2 knockdown prevents cerebrovascular remodeling via inhibition of the Wnt/beta-catenin signaling pathway. Cell Mol Biol Lett. 2018;23:29. https://doi.org/10.1186/s11658-018-0095-z.
Grodin JL, Mullens W, Dupont M, Taylor DO, McKie PM, Starling RC, et al. Hemodynamic factors associated with serum chloride in ambulatory patients with advanced heart failure. Int J Cardiol. 2018;252:112–6. https://doi.org/10.1016/j.ijcard.2017.11.024.
Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, et al. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157(2):447–58. https://doi.org/10.1016/j.cell.2014.03.024.
Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, et al. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344(6184):634–8. https://doi.org/10.1126/science.1252826.
Feihl F, Liaudet L, Waeber B. The macrocirculation and microcirculation of hypertension. Curr Hypertens Rep. 2009;11(3):182–9.
Chiron D, Martin P, Di Liberto M, Huang X, Ely S, Lannutti BJ, et al. Induction of prolonged early G1 arrest by CDK4/CDK6 inhibition reprograms lymphoma cells for durable PI3Kdelta inhibition through PIK3IP1. Cell Cycle. 2013;12(12):1892–900. https://doi.org/10.4161/cc.24928.
Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Gerontol Ser A Biol Sci Med Sci. 2005;60(3):293–300.
Liu F, Yang X, Geng M, Huang M. Targeting ERK, an Achilles’ Heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B. 2018;8(4):552–62. https://doi.org/10.1016/j.apsb.2018.01.008.
Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, et al. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274(32):22699–704.
Sardini A, Amey JS, Weylandt KH, Nobles M, Valverde MA, Higgins CF. Cell volume regulation and swelling-activated chloride channels. Biochem Biophys Acta. 2003;1618(2):153–62.
Cheng G, Kim MJ, Jia G, Agrawal DK. Involvement of chloride channels in IGF-I-induced proliferation of porcine arterial smooth muscle cells. Cardiovasc Res. 2007;73(1):198–207. https://doi.org/10.1016/j.cardiores.2006.10.012.
Eechoute K, Sparreboom A, Burger H, Franke RM, Schiavon G, Verweij J, et al. Drug transporters and imatinib treatment: implications for clinical practice. Clin Cancer Res. 2011;17(3):406–15. https://doi.org/10.1158/1078-0432.CCR-10-2250.
Yang C, He L, Chen G, Ning Z, Xia Z. LRRC8A potentiates temozolomide sensitivity in glioma cells via activating mitochondria-dependent apoptotic pathway. Hum Cell. 2019;32(1):41–50. https://doi.org/10.1007/s13577-018-0221-2.
Sorensen BH, Nielsen D, Thorsteinsdottir UA, Hoffmann EK, Lambert IH. Downregulation of LRRC8A protects human ovarian and alveolar carcinoma cells against cisplatin-induced expression of p53, MDM2, p21Waf1/Cip1, and Caspase-9/-3 activation. Am J Physiol Cell Physiol. 2016;310(11):C857–73. https://doi.org/10.1152/ajpcell.00256.2015.
Stuhlmann T, Planells-Cases R, Jentsch TJ. LRRC8/VRAC anion channels enhance beta-cell glucose sensing and insulin secretion. Nat Commun. 2018;9(1):1974. https://doi.org/10.1038/s41467-018-04353-y.
Bao J, Perez CJ, Kim J, Zhang H, Murphy CJ, Hamidi T, et al. Deficient LRRC8A-dependent volume-regulated anion channel activity is associated with male infertility in mice. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.99767.
Morgan DO. Principles of CDK regulation. Nature. 1995;374(6518):131–4. https://doi.org/10.1038/374131a0.
Choi H, Ettinger N, Rohrbough J, Dikalova A, Nguyen HN, Lamb FS. LRRC8A channels support TNFalpha-induced superoxide production by Nox1 which is required for receptor endocytosis. Free Radic Biol Med. 2016;101:413–23. https://doi.org/10.1016/j.freeradbiomed.2016.11.003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, J., Xu, F. & Zhang, J. Inhibition of angiotensin II-induced cerebrovascular smooth muscle cell proliferation by LRRC8A downregulation through suppressing PI3K/AKT activation. Human Cell 32, 316–325 (2019). https://doi.org/10.1007/s13577-019-00260-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-019-00260-6