Abstract
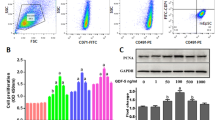
The objective of the study was to explore the influence of junctional adhesion molecule A (JAM-A) gene decoration on proliferation and differentiation of human epidermal stem cells (hEpSCs). JAM-A gene and JAM-A interference gene lentivirus eukaryotic expression vectors were established. The recombinant lentivirus was introduced into hEpSCs to observe and detect viral transfection by fluorescence microscopy and Western blot, respectively. After confirmation of successful introduction of the target gene, cell growth curves were mapped out by cytometry to detect cell proliferation in different groups. The expression of hEpSCs labeled molecules was detected by immunofluorescence, and cell safety was detected by teratoma test in all groups. (1) Fluorescence microscopy showed that in the JAM-A over-expression (JAM-Aov EpSCs) group, the green fluorescence was mainly distributed in the cell membrane; in the JAM-A interference (JAM-Akd EpSCs) group and blank vector (GFP EpSCs) group, all cell bodies were luminous. Western blot showed that JAM-A protein was up-regulated in JAM-Aov EpSCs and down-regulated in JAM-Akd EpSCs. (2) Growth curves showed that hEpSCs entered the quick-growing phase 4 days after inoculation and reached the platform phase at day 7. JAM-Aov EpSCs proliferated more slowly than GFP EpSCs, while JAM-Akd EpSCs proliferated significantly faster than GFP EpSCs. (3) Immunofluorescence showed that the expression of transient amplification epidermal marker keratin 14, hEpSCs marker keratin I9 and β-integrin was down-regulated in JAM-Akd EpSCs group as compared to that in the GFP EpSCs group, and the expression of epidermal terminal differentiation marker K10 was negative in the JAM-Akd EpSCs group. There was no significant difference in the expression of specific molecules between JAM-Aov EpSCs and hEpSCs. (4) The result of teratoma test was negative in all groups. The proliferative ability of hEpSCs was increased markedly after down-regulation of JAM-A. Cells presented initial differentiation, but retained their stem cell characteristics without evidence of tumorigenesis.







Similar content being viewed by others
References
Martìn-Padura I, Lostaglio S, Schneemann M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;13(1):117–27.
Liu Y, Nusrat A, Schnell FJ, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113:2363–74.
Mandell KJ, McCall IC, Parkos CA. Involvement of the junctional adhesion molecule-1 (JAM-A) homodimer interface in regulation of epithelial barrier function. J Biol Chem. 2004;279(16):16254–62.
Mandell KJ, Babbin BA, Nusrat A, et al. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on β1 integrins and Rap1 activity. J Biol Chem. 2005;280:11665–74.
Prota AE, Campbell JA, Schelling P, et al. Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc Natl Acad Sci USA. 2003;100(9):5366–71.
Ivanov AI, Nusrat A, Parkos CA. The epithelium in inflammatory bowel disease: potential role of endocytosis of junctional proteins in barrier disruption. Novartis Found Symp. 2004;263:115–24 (discussion 124-32, 211-8).
Peddibhotla SS, Brinkmann BF, Kummer D, et al. Tetraspanin CD9 links junctional adhesion molecule-A to αvβ3 integrin to mediate basic fibroblast growth factor-specific angiogenic signaling. Mol Biol Cell. 2013;24(7):933–44.
Lakshmi SP, Reddy AT, Naik MU, et al. Effects of JAM-A deficiency or blocking antibodies on neutrophil migration and lung injury in a murine model of ALI. Am J Physiol Lung Cell Mol Physiol. 2012;303(9):L758–66.
Tsuruta Y, Pereboeva L, Glasgow JN, et al. Reovirus sigma1 fiber incorporated into adenovirus serotype 5 enhances infectivity via a CAR-independent pathway. Biochem Biophys Res Commun. 2005;335(1):205–14.
Severson EA, Lee WY, Capaldo CT, et al. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol Biol Cell. 2009;20:1916–25.
Nava P, Capaldo CT, Koch S, et al. JAM-A regulates epithelial proliferation through Akt/b-catenin signaling. EMBO Rep. 2011;12:314–20.
Cotsarelis G, Kaur P, Dhouailly D, et al. Epithelial stem cells in the skin: definition, markers, localization and functions. Exp Dermatol. 1999;8(1):80–8.
Watt FM, Jensen KB. Epidermal stem cell diversity and quience. EMBO Mol Med. 2009;1(5):260–7.
Suzuki A, Sekiya S, Gunshima E, et al. EGF signaling activates proliferation and blocks apoptosis of mouse and human intestinal stem/progenitor cells in long-term monolayer cell culture. Lab Invest. 2010;90(10):1425–36.
Stoyanova T, Goldstein AS, Cai H, et al. Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via β-catenin signaling. 2012; 6(20):2271–85
Zhu B, Smith J, Yarmush ML, et al. Microfluidic enrichment of mouse epidermal stem cells and validation of stem cell proliferation in vitro. Tissue Eng Part C: Methods. 2013;19(10):765–73.
Abburi C, Prabhakar S, Kalra J, et al. Vascular endothelial growth factor (VEGF) induced proliferation of human fetal derived ciliary epithelium stem cells is mediated by jagged-N cadherin pathway. Curr Neurovasc Res. 2013;10(2):93–102.
Plikus MV, Gay DL, Treffeisen E, et al. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol. 2012;23(9):946–53.
Rizzi SC, Upton Z, Bott K, et al. Recent advances in dermal wound healing: biomedical device approaches. Expert Rev Med Devices. 2010;7(1):143–54.
Degen KE, Gourdie RG. Embryonic wound healing: a primer for engineering novel therapies for tissue repair. Birth Defects Res C Embryo Today. 2012;96(3):258–70.
Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25(1):73–8.
Lau K, Paus R, Tiede S, et al. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol. 2009;18(11):921–33.
Gauglitz GG, Jhke MG. Combined gene and stem cell therapy for cutaneous wound healing. Mol Pharm. 2011;8(5):1471–9.
Lu CP, Polak L, Rocha AS, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150(1):136–50.
Mathe Z, Dupraz P, Rinsch C, et al. Tetracycline-regulated expression of VEGF-A in beta cells induces angiogenesis: improvement of engraftment following transplantation. Cell Transplant. 2006;15(7):621–36.
Naik MU, Mousa SA, Parkos CA, et al. Signaling through JAM-1 and alphavbeta3 is required for the angiogenic action of bFGF: dissociation of the JAM-1 and alphavbeta3 complex. Blood. 2003;102(6):2108–14.
Acknowledgments
This work was supported by National 973 Project of China (No. 2011CB965101) and China Postdoctoral Science Foundation (No. 2012M512061).
Conflict of interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Wu, T. Zhou and X. Guo are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, T., Wu, M., Guo, X. et al. RNA interference mediated JAM-A gene silencing promotes human epidermal stem cell proliferation. Human Cell 28, 73–80 (2015). https://doi.org/10.1007/s13577-013-0087-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-013-0087-2