Abstract
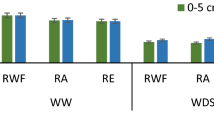
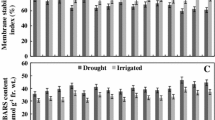
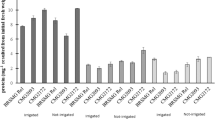
Drought stress is a serious threat which decreases crop production. The present investigation was taken up to study the effect of drought stress at vegetative and reproductive stages on potential biochemical and enzymatic responses were investigated in two rice cultivars Rashi (tolerant) and Swarna (sensitive). Here, we observed significant changes in the biochemical and enzymatic responses between control and treatments as well as between the genotypes. Activity of catalase, peroxidase and superoxide dismutase (SOD), and glutathione reductase were also found to be different among the rice cultivars. Moreover, phenol content and activity of superoxide dismutase, catalase and glutathione reductase were increased significantly under drought stress in Rashi compared to Swarna. Nitrogen assimilatory enzymes such as nitrate and nitrite reductase (NiR) were sensitive to drought stress and both the genotype showed decreased activity at reproductive stage. Catalase activity was significantly higher at controlled reproductive (11.72%) and vegetative stage (4.14%) compared to Swarna. Rashi also showed an elevated SOD activity in controlled reproductive (180.55%) and vegetative stage (7.67%) compared to Swarna. Similarly, highest NiR activity found in Swarna (17.03%) compared to Rashi. Highest nitrate reductase activity was found in Rashi (10.87%) compared to Swarna. Rashi showed significantly higher chlorophyll content in vegetative stage and decreased in reproductive stage (26.33%). The improved performance of drought tolerant genotype (Rashi) was associated with more efficient biochemical factors under conditions of stress compare to drought sensitive genotype (Swarna).
Similar content being viewed by others
Abbreviations
- SOD:
-
Superoxide dismutase
- NiR:
-
Nitrite reductase
- GR:
-
Glutathione reductase
References
Akhtar MR (2006) impact of resource conservation technologies for sustainability of irrigated agriculture in Punjab, Pakistan. J Agric Res 44:239–257
Asada K (1994) Production and action of active oxygen species in photosynthetic tissues. In: Foyer C, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, pp 77–100
Awika JM (2011) Major cereal grains production and use around the world. ACS Symp 1089:1–13
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chinnusamy V, Zhu JK, Sunkar R (2010) Gene regulation during cold stress acclimation in plants. Methods Mol Biol 639:39–55
Chozin MA, Lubis I, Junaedinand A, Ehara H (2014) Some physiological character responses of rice under drought conditions in a paddy system. J Int Soc Southeast Asian Agric Sci 20(1):104–114
Cruz CMH (2008) Drought stress and reactive oxygen species. Plant Signal Behav 3:156–165
Daryanto S, Wang L, Jacinthe PA (2017) Global synthesis of drought effects on cereal, legume, tuber and root crops production: a review. Agric Water Manag 179:18–33
Dubey S, Pallavi SR (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46:209–221
Elstner EF (1991) Mechanisms of oxygen activation in different compartments of plant cells. In: Pell EJ, Steffen KL, Rockville MD (eds) Active oxygen/oxidative stress and plant pell metobolism. American Society of Plant Physiologists, Rockville, pp 13–25
Emam MM (2012) Antioxidant defence system of black rice grown under drought. Egypt J Exp Biol Bot 8(2):261–269
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147
Farooq M, Hussain M, Siddique KHM (2014) Drought stress in wheat during flowering and grain-filling periods. Crit Rev Plant Sci 33:331–349
Flores P, Botella MA, Martinez V, Cerda A (2002) Ionic and osmotic effects on nitrate reductase activity in tomato seedlings. J Plant Physiol 156:552–557
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants physiological biochemical and molecular characterization. Int J Genome 130:2129–2141
Hageman RH, Reed AJ (1980) Nitrate reductase from higher plants. Methods Enzymol 69:270–280
Hatfield JL, Prueger JH (2015) Temperature extremes: effect on plant growth and development. Weather Clim Extrem 10:4–10
Hiscox JD, Tsraelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Lea PJ (1993) Nitrogen metabolism. In: Lea PJ, Leegood RC (eds) Plant biochemistry and molecular biology. Wiley, New York, pp 155–180
Lesk C, Rowhani P, Ramankutty N (2016) Influence of extreme weather disasters on global crop production. Nature 529:84–87
Madhusudhan R, Ishikawa T, Sawa Y, Shiqeoka S, Shibata H (2003) Characterization of an ascorbate peroxidase in plastids of tobacco BY-2 cells. Physiol Plant 117:550–557
Mahdi DZ, Hemantaranjan A, Panday SK (2007) Antioxidative response of mung bean (Vigna radiate L.) to salt stress. Legume Res 30:57–60
Mallick N, Mohn FH (2000) Reactive oxygen species: response of algal cells. J Plant Physiol 157:183–193
Matiu M, Ankerst DP, Menzel A (2017) Interactions between temperature and drought in global and regional crop yield variability during 1961–2014. PLoS ONE 12:e0178339
Mavis RD, Stellwagen E (1968) Purification and subunit structure of glutathione reductase from bakers’ yeast. J Biol Chem 243:809–814
McGuire S, WHO, World Food Programme, and International Fund for Agricultural Development (2012) The State of Food Insecurity in the World 2012. Economic growth is necessary but not sufficient to accelerate reduction of hunger and malnutrition. Advances in Nutrition, vol 4. FAO, Rome, pp 126–127
Miller G, Suzuki N, Ciftci YS, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mithofer A, Boland W (2007) Insects feeding on plants: rapid signals and responses preceding the induction of phytochemical release. Phytochemistry 68:2946–2959
Monakhova OF, Chernyadev II (2002) Protective role of kartolin-4 in wheat plants exposed to soil drought. Appl Environ Microbiol 38:373–380
Montano M, Sukegawa K, Kato Z, Carrozzo R, Natale PD, Christensen E, Orii KO, Orii T, Kondo N, Tomatsu S (2007) Effect of ‘attenuated’ mutations in mucopolysaccharidosis IVA on molecular phenotypes of N-acetylgalactosamine-6-sulfate sulfatase. J Inherit Metab Dis 30:758–767
Nakashima K, Ito Y, Yamaguchi-Shinozaki K (2009) Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol 149:88–95
Nayyar H, Gupta D (2006) Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Environ Exp Bot 58:106–113
Oaks A (1994) Primary nitrogen assimilation in higher plants and its regulation. Can J Bot 72:739–750
Parry ML, Canziani OF, Palutikof JP, Linden PJVD, Hanson CE (2007) Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. In: Leal Filho W (ed) Encyclopedia of language and linguistics, vol 12. Elsevier, New York, pp 171–175. Wissenschaften Hamburg, Forschungs- und Transferzentrum, Hochschule für Angewandte, Hamburg, Germany
Rachana D, Narinder K, Anil Kumar G (2012) Potential of antioxidant in depicting drought tolerance of wheat (Triticum aestivum L.). Indian J Biochem Biophys 49:257–265
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110
Sadasivam S, Manikam A (1992) Biochemical methods for agricultural sciences. Wiley Eastern Limited, New Delhi, pp 150–151
Sarsenbaev KN, Abiyev SA, Kozhamzhaova ZY, Bazarbayeva Z (2013) Effect of leaf rust on the molybdenum containing enzyme activity in spring wheat. World Appl Sci 25:30–32
Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Tas S, Tas B (2007) Some physiological responses of drought stress in wheat genotypes with different ploidity in Turkiye. World J Agric Sci 3:178–183
Tilman D, Balzer C, Hill J, Befort BL (2011) From the cover: global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA 108:20260–20264
Ushimaru T, Ogawa K, Ishida N, Shibasaka N, Kanematsu S, Asada K, Tsuji H (1995) Changes in organelle superoxide dismutase isozymes during air adaptation of submerged rice seedlings: differential behaviour of isozymes in plastids and mitochondria. Planta 112:606–613
Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G (2003) Scavenging of reactive oxygen species in NaCl stressed rice (Oryza sativa L.)—differential response in salt tolerant and sensitive varieties. Plant Sci 165(6):1411–1418
Wahab MA (2006) The efficiency of using saline and fresh water irrigation as alternating methods of irrigation on the productivity of Foeniculum vulgare Mill subsp. vulgare var. vulgare under North Sinai conditions. Res J Agric Biol Sci 2:571–577
Wray JL, Fido J (1990) Nitrate reductase and nitrite reductase. In: Lea PJ (ed) Methods in plant biochemistry, enzymes of primary metabolism, vol 3. Academic Press, London, pp 241–256
Yamasaki H, Sakihama Y, Takahashi S (1999) An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci 4:128–129
Acknowledgements
We thank Directorate of Research, University of Agricultural Sciences, Dharwad, India for the financial support, the fellowship and Dr. B. N. Aravind Kumar for his guidance in conducting pot experiments. We extend thanks to Dr. K. V. Ashalatha for her guidance in statistical analysis. The resistant cultivar Rashi will be used for breeding further breeding program to come up with a better resistant variety. The information obtained from the present investigation will be utilized to design and conduct molecular experiments involving marker assisted selection, transcriptome analysis and stress response protein identification
Author information
Authors and Affiliations
Contributions
Mahadev: The graduate student has carried out the research work as a part of her M.Sc. Programme. KK: Principle Investigator, who has conceptualized, designed the research programme and arranged for all the infrastructural facilities required for the research programme. AK professor of Agronomy has guided and helped us in conducting pot culture studies in poly house.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumbar, M., Mirajkar, K.K. & Arvind, K. Phytochemical response in rice (Oryza sativa L.) genotype during the vegetative and reproductive stage under drought stress and non-stress conditions. J. Plant Biochem. Biotechnol. 30, 1–12 (2021). https://doi.org/10.1007/s13562-020-00555-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-020-00555-y