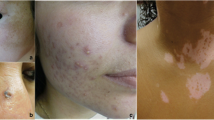
Abstract
Background
Dermatomyositis (DM) is a rare and severely debilitating autoimmune disease that can affect children and adults; however, there is little understanding of the patient-reported experience and uncertainty around validated clinical outcomes assessments (COAs) that could measure changes in the condition during clinical trials of new treatments.
Objectives
The aim of this study was to understand the patient experience of DM, with a focus on its cutaneous manifestations, to describe the patient experience and determine the suitability of existing COA measures.
Methods
Adult (≥ 18 years) patients (N = 28) with severe active cutaneous manifestations of DM were interviewed. In the 90-min interviews, open-ended questions and probes were used to elicit descriptions of key clinical manifestations and patients’ experiences of DM, including the symptoms and impacts on their daily lives and wellbeing.
Results
Patients reported 13 different skin manifestations of DM. The most common were rash (n = 28, 100%), itch (n = 28, 100%), dry skin (n = 23, 82%), and swelling of the skin (n = 17, 61%). The head and face, followed by hands, were perceived as the most bothersome body areas affected by skin manifestations, because they are exposed and visible to themselves and other people. All patients (n = 28, 100%) reported at least one impact of DM, which varied greatly between patients, but included emotional, psychological, cognitive, and physical impacts, and those affecting daily life, such as work and sleep. Over half of the patients (n = 19, 67%) reported that their daily activities were impacted by DM.
Conclusions
The qualitative interviews with patients revealed that the presentation of DM manifestations is highly variable but affects patients’ emotional wellbeing, physical activities, and daily life significantly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The quality of life (QOL) of patients with dermatomyositis (DM) is greatly affected, specifically their emotional wellbeing. This is mainly due to the cutaneous (skin) manifestations (signs and symptoms) experienced. |
There is a paucity of recently published research that clearly documents the experiences of adults who have been diagnosed with DM, specifically what is important to them regarding cutaneous manifestations. |
The lack of qualitative information makes it challenging to ensure clinical trials of DM are incorporating relevant clinical outcome assessment (COA) measures as endpoints. |
What was learned from the study? |
This study provides insightful knowledge into the experiences of adults with DM (aged ≥ 18 years at diagnosis) and active skin manifestations and how they are impacted by the condition. The results indicate that DM affects people’s QOL significantly, and this information is presented along with supporting patient quotes. In addition, this study demonstrates a clear unmet need for a novel drug that can provide patients with a resolution to their cutaneous manifestations. |
Patients QOL is impacted via the different manifestations they experience due to DM. Cutaneous signs and symptoms especially seem impactful to patients’ emotional wellbeing, although other impacts spanning many other areas of patients’ lives were reported. |
DM is a debilitating disease with limited treatment options, and the current study may lead to the identification or development of meaningful cutaneous COA measures for DM. |
Introduction
Dermatomyositis (DM) is a severe, rare, heterogenous autoimmune disease which typically leads to muscle weakness, rashes, and variable involvement of other organs including cardiac manifestations, arthritis, and interstitial lung disease (ILD) [1]. The characteristic rash includes Gottron’s papules or sign and heliotrope rash; however, the full spectrum includes rashes throughout the body [2]. The cutaneous manifestations of DM (specifically the erythematous rash) can be detrimental to patient quality of life (QoL), especially to emotional wellbeing [3]. It has been reported that the amount of cutaneous involvement that a patient has correlates directly with the impact on their QoL [4, 5]. Data from existing trials report patients experience a positive QoL change even when small improvements in skin disease have been noted on certain outcome measures [6]. Although rash appears to be the most impactful concept to patients and widely experienced, several other cutaneous and non-cutaneous manifestations can present. These include painful skin ulceration, pruritis, and muscle weakness [7, 8].
DM can affect children (where the disease is known as juvenile dermatomyositis [JDM]) and adults, most commonly occurring in children aged 5–15 years and adults aged 40–60 years [9, 10]. DM affects women more than men and is associated with high rates of morbidity and mortality, including increased risk of malignancy [9, 11,12,13,14]. Currently, DM has no known cure and very limited treatment options with proven safety and efficacy in randomized controlled trials and is frequently treatment-refractory [15].
To conduct patient-focused drug development (PFDD), it is essential that the patient experience of the condition is fully understood. This information underpins the selection, validation, and development of suitable clinical outcome assessments (COAs) to measure changes in the condition during clinical trials of novel treatments. Conducting qualitative research with patients provides an understanding of disease from the patient perspective and contributes more robust learnings, which consequently should help to engage patients [16]. Although many papers report on the experience of patients with JDM, there is a paucity of relevant, recent publications on how adults with DM feel and function [17].
The aim of this study was to understand the adult patient experience of DM, with a focus on the cutaneous manifestations, via in-depth qualitative interviews with patients and expert DM clinicians. This article reports on the results of patient interviews (N = 28).
Methods
This qualitative interview study was designed in line with the FDA patient-reported outcome (PRO) Guidance for industry [18] and PFDD guidance [19,20,21,22]. The reporting of the study outlined in this manuscript abided by the Standards for Reporting Qualitative Research (SRQR) guidelines. [23].
A targeted literature review was conducted, prior to conducting qualitative interviews with patients, to identify published qualitative studies involving patients with DM. The results of the review are not presented in this article but were used to produce a draft conceptual model that visually represented the patient experience of DM and was used to guide development of the study materials.
Patient Interviews
Recruitment and Sampling Strategy
Individual 90-min qualitative interviews were conducted with (N = 28) patients (aged ≥ 18 years) with a clinician-confirmed diagnosis of DM in the US and severe active cutaneous manifestation in the past 12 months. The patient inclusion and exclusion criteria are provided in the Supplementary Material (Table S1). Patients were recruited through a specialist recruitment agency and patient advocacy group (Myositis Support and Understanding). Purposive sampling was used to target a demographically diverse sample that was representative in terms of sex (50% female), education (33% high school diploma or less), and ethnicity (33% non-Caucasian).
Ethical Approval and Informed Consent
Prior to commencing the interviews, ethical approval was sought from the WCG Independent Review Board (IRB) in the US (IRB study number: 1316795; IRB tracking number: 20214484; approval date: 8 September 2021). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Written informed consent was obtained from patients prior to their interview, and they were compensated for their time.
Interview Procedure
A semi-structured interview guide [example questions provided in the Supplementary Material (Table S2)] was used to explore the objectives in the 90-min interview. The concept elicitation section of the interview included open-ended questions and probes to explore patients’ experiences of DM, including the signs and symptoms they experienced and the associated impacts of DM on their daily lives and wellbeing.
Analysis
Audio recordings from the patient interviews were transcribed verbatim. De-identified transcripts were coded using semantic, qualitative, directed content analysis techniques [24] aided by ATLAS.ti Version 9 computer-assisted data analysis software, which facilitates the coding and organization of data. Quantifiable data, e.g., demographic and clinical characteristics, were descriptively summarized (e.g., means and frequencies) using Microsoft Excel. Using this approach, we sought to understand the patient experience of DM, focusing on their feelings, functions, and lived experiences. Frequency of reporting was calculated for key concepts and themes. The following process of qualitative analysis was followed:
-
Immersion in the study: Researchers had a deep understanding of the study and discussed overarching analytic ideas in relation to the study objectives.
-
Coding: Each transcript was read by a researcher and descriptive codes were assigned to quotes/text within the publications.
-
Iterative review of codes: The initial fit of the codes to the data was revisited throughout the coding process. As new codes emerged, previous transcripts were reviewed and new codes applied as appropriate. Codes were also merged together or split into more detailed codes.
-
Defining and refining concepts: The research team met to discuss their findings and reflect on the codes applied, making further revisions to ensure coding was consistent across multiple coders. Codes were then organized to identify, define, and refine relevant concepts and domains.
-
Reporting: Final concepts and domains were reported alongside quotes. Counts were used where appropriate.
Unique identifiers (IDs) were used to anonymize reporting of the data. Patient IDs were as follows: interview order-sex-age-presence of skin manifestations (S) or skin and muscle (SM) manifestations. For example, 08-M-49-SM denoted the eighth patient, who was male, aged 49, and had skin and muscle manifestations.
Conceptual Saturation
Analysis of conceptual saturation was performed using spontaneously elicited concepts from the interviews. Conceptual saturation is defined as the point at which no new concept-relevant information emerges [25]. The analysis was performed to determine whether all key concepts related to the patient experience of DM had been captured. In line with industry guidance, the saturation analysis was planned a priori and was assessed by dividing transcripts into equally numbered sets based on the chronological order of interview completion and comparing the concepts that emerged in each set [26].
Results
Patient Interviews
Sample
Twenty-eight patients (mean age, range: 49.6 years, 18.0–68.0) participated in the study. Patients' clinical and demographic characteristics are presented in Table 1. Consistent with statistics on DM prevalence [27], 68% of patients (n = 19) were female. The mean (range) time since DM diagnosis was 3.8 years (0.2–23.1). All patients had DM skin manifestations (n = 28, 100%), and 57% (n = 16, 57%) also experienced muscle manifestations. The study sample consisted of patients who had a range of different skin tones, which allowed the research team to explore whether DM cutaneous manifestations differed visually between patients.
Overview of Patient Reported Signs and Symptoms
Patients spontaneously reported 13 different skin signs/symptoms of DM. The most frequently reported symptoms were rash (n = 28, 100%), itch (n = 28, 100%), dry skin (n = 23, 82%), and swelling of the skin (n = 17, 61%). Other frequently reported symptoms included bumps (n = 14, 50%), sores (n = 13, 46%), and nail-bed changes (n = 13, 46%) (Supplementary Material, Table S3).
Sixteen (57%) patients reported that they had experienced DM-related muscle symptoms. Of the eight different muscle symptoms reported, the most common (n = 16 patients, 100%) was muscle weakness, followed by muscle pain/aches/soreness (n = 11, 69%).
Rash
Most patients described the appearance of their rash as red (n = 25/28, 89%). Other commonly used descriptors were bumpy (n = 20/28, 71%), dry (n = 15/28, 54%), and purple (n = 14/28, 50%). Descriptions of rash given by Caucasian and non-Caucasian patients were largely aligned. The top three descriptors of rash used by Caucasian and non-Caucasian patients were red, bumpy, and dry. Other descriptors used included “flaky,” “thick,” and “rough”:
-
“I developed a severe bright red, err, shawl rash on my back and my chest. Um, I’m not a very tanned person, but this was extreme—it was, like, almost like a—to a violet r-red on my chest and back and it was very, very pronounced and I had very, very pronounced redness in, in my face, within the, the, the lines of—on e-either side of my nose, across my face and my entire face would turn bright red, as part of some of the, um, err, um—a, a part of it progressing.” (03-M-50-S)
-
“Sometimes it’s bumpy and sometimes it’s flat. I’ve noticed on my face consistently it’s been flat, um, from onset upwards and also, like, on my eyelid area, it’s, like, the flat type of reddish, purplish color.…But the face is definitely the red and—I get the red and the purple.” (27-F-45-SM)
-
“It’s very, very, like, dry, rough, dry and no – not feeling moisturized.…Yeah, we’ve got the, the itchiness is definitely around the rash area. Um, I don’t—not any itch and, like, you know, bothering my feet or something that’s not in the area where it is.” (14-F-59-SM)
All patients were asked whether the appearance of their rash differed across different body areas or whether the rash appearance remained consistent across body areas. Two thirds (n = 12/18, 66%) reported differences across body areas, whereas the remaining patients (n = 6/18, 33%) reported no such differences. Patients were also asked to describe the area of their body that their rash affected. The most common body areas affected by rash are outlined in Fig. 1.
Itch
Nearly all patients (n = 27/28, 96%) had experienced DM-related itch. Eight (n = 8/27, 30%) reported that the itch was associated with pain if the skin was scratched too much. When asked about the location of itching, areas of the body affected included the hands (n = 12/27, 44%), head (n = 12/27, 44%), elbows (n = 5/27, 19%), and legs/knees (n = 5/27, 19%). Itch was typically associated with an area of rash on the skin.
-
“Yeah, we’ve got the, the itchiness is definitely around the rash area. Um, I don’t—not any itch and, like, you know, bothering my feet or something that’s not in the area where it is. So, it’s the I—err, itch is around the rash, and I think it’s—the skin is upset, annoyed, and then when you see it, you like to pester it and it could, you know, create irritation there and then it makes the itch worse, so you must use, like, a cream.…I mean, at times, yes, I’ve done some serious scratching and I’ve broken the skin barrier and got some scabs and some scars.” (14-F-59-SM)
-
“The pain from itching has happened when I rip a scab or rip it. Um, not pain that is throbbing, like you’ve stub your toe and it’s—hurts, like, in the second and you can’t think of anything else. It’s more, the pain is, um, produced by my actions.” (24-F-49-SM)
Dry skin
The majority of patients (n = 23/28, 82%) reported that they experienced dry skin due to their DM. Descriptions included flaky (n = 18/23, 78%) or scaly (n = 8/23, 35%). Five patients (22%) described the appearance as crusty and five (22%) reported that their dry skin would occasionally crack. The most commonly reported body areas to be affected were the hands (n = 14, 61%), of which the knuckles were mentioned as being particularly prone, and the head/face (n = 9, 39%).
“My hands are at its worst. They’re very—it can get, at times, very scaly, really scaly, kind of, nasty to look at. Um, underneath my eyes, um, definitely they can get puffy and, um, and dark. I look like I have raccoon eyes, almost, they’re dark and red, err, a little puffy. Um, and then my legs has got it the least. It’s just, you know, dry and flaky at times, err, um, nothing major, just noticeable, a little bit irritating, itchy.” (22-M-47-SM)
Swelling of the skin
Over half of the patients (n = 17, 61%) reported they had experienced swelling of their skin. Patients used terms including “puffy” and “bulbous” to describe the appearance. Patients reported the swelling as being bothersome for them.
“Yeah, mainly my, um, my hands. It’ll be always in my fingers, you know, my fingers will swell up. Um, you know, it’ll, it’ll feel like everything is tight and the ankles every now and then…Um, it’s bothersome, just because it, it – especially when it’s in my hands, ‘cause it, it, it makes it hard to move my hands. It makes ‘em, you know—they, they feel stiff.” (15-F-47-SM)
Symptom variability
Symptoms were variable for most patients (25/28, 89%). The term “flares” was used to describe the time when symptoms worsened, but patients also used the terms “bouts” and “outbreaks.” The duration of flares ranged from a few days to 3 months, with different amounts of time in between (from days to months). Different reasons and triggers were suggested by patients as a cause for their flares, including being in the sun (n = 4, 16%), seasonal changes (n = 4, 16%), diet, stress, and certain soaps (all n = 1).
-
“Um, well, sometimes, yeah, depending on, I feel like the weather or, um, you know, I, I, I don’t know, or just the, the disease itself. But I believe that, err, weather may have something to do with it too, but it does, err, come and go, yes.” (11-F-39-S)
-
“I think it’s, err, irritants. I think it’s weather, is a big part of it. I think it just adds to the condition, you know, when it’s a whole out, if I’m not trying to moisturize, you know, on top of everything else while it’s cold, or if, err, maybe, err, you know, soap or, err, some kind of chemical might irritate it. Of course, if I’m, let’s say, I’m cleaning for instance, so, I noticed that can do it with certain things. Certain brands of, err, products can do that. Um, but, yeah, I think it’s all based on what I’m doing, really, and, and weather.” (22-M-47-SM)
Most bothersome body area
All patients (n = 28, 100%) were asked to name their top three most bothersome body areas affected by skin symptoms. Head and face were most commonly ranked as the most bothersome (n = 16, 57%), closely followed by hands (n = 9, 32%). Patients explained that these were the most bothersome because they are exposed and visible to themselves and others.
-
I: Okay. What makes the face the most bothersome area? “Yes, first of all because of the itching, and the color, and, you know…and, um, dry—with my family or my, err, customers, whatever, the public, err, it, err, it, i-it, it, it makes their attention to me and, err, they look at me, so, um, err, it bothers me from inside and outside.” (18-F-57-SM)
-
“Yeah, with, with the first one, because people can see my hands, too. That’s two things it’s obvious to the people when I have a problem, so that’s how it is. Hands is the same. I can’t go to supermarket, because if I go to cashier, they, they don’t like that—to see my hands like this. So, I wearing some gloves now and things that you can wear the gloves, um, but—and masks, so it’s covered. That’s the thing why you putting in the first place, because this is—beside that bothers as a problem, skin problem, it bothers me people can see it. It’s, kind of, embarrassing, little bit.” (25-F-68-SM)
In a separate ranking exercise, patients were also asked which body area affected by skin manifestations would be most important for a new treatment to treat/improve. Aligned with the results from the most bothersome body area task, the head and face (n = 19, 68%) were reported as the most important areas to improve, followed by hands (n = 11, 39%).
Descriptions of meaningful improvement
Most patients (n = 19, 68%) reported that they would like their skin symptoms to be completely cured, citing change in the core skin symptoms of rash, itch, and dry skin. Others (n = 9, 32%) reported that they would like a new treatment to reduce the appearance and/or feeling of their skin symptoms or the frequency of which they experienced symptom flares.
-
“…well, first, first and foremost, I, I, I, I hope for a treatment that will put this into remission.…Um, err, the, err, the, the primary th-thin-thing for me is if I can’t go into a remission, is to get rid of the first, um, and then second would be the look of what the—um, the physical appearance of the scaly skin, the red-redness and everything else, and, and um, so if they can make the, the pain go away…(one) pain, (two) looks, um, and making that go.” (03-M-50-S)
-
“Um, the—well, the improvement would be that it is gone. Like, it’s—um, I might be able to—I’m not looking for it to be, like, cosmetic, where the skin is just perfect and all this. To me, like, my face, the color and what people can see, a rash, kind of, just like, you know, it’s, um, it’s not the skin tone of your skin. Um, it, it, it, it, it exacerbates it, and it shows up, so it’s apparent. If it can reduce that and just look flush, like your skin, I—that, to me, is a win.” (04-F-52-S)
Overview of Patient Reported Impacts
All patients (n = 28, 100%) reported at least one impact of DM. The impacts varied greatly between patients, but included emotional, psychological, and/or cognitive impacts, physical impacts, and those affecting daily life, such as work/school and sleep (Supplementary Material, Table S4).
All patients (n = 28, 100%) reported experiencing emotional, psychological, and/or cognitive impacts of living with DM. The most frequently reported emotional impacts were feeling “upset” (n = 16, 57%), “frustrated” (n = 15, 54%), “worry” (n = 12, 43%), and “embarrassed” (n = 12, 43%). Two-thirds of patients (n = 19, 68%) had experienced stigma from other people in relation to their DM symptoms.
Physical impacts of living with DM were reported by 54% of patients (n = 15/28) and included difficulties with activities such as lifting objects (n = 13/28, 46%), getting up from a seated position (n = 4/28, 14%), walking or climbing stairs (n = 3, 11%), gripping/holding on to things (n = 3/28, 11%), and difficulty swallowing (n = 3/28, 11%).
Many patients (n = 19, 67%) reported that their daily activities were impacted by DM. The most frequently reported impact was on patients’ ability to complete household chores (n = 8, 29%), which was often affected by muscle weakness and pain (n = 6/8, 75%). Skin symptoms also affected patients’ ability to complete chores because of skin irritation from household cleaners (n = 4/8, 50%). One-fifth of patients (n = 6/28, 21%) experienced difficulties with dressing and grooming due to skin rashes (n = 2/6, 33%) and muscle pain or weakness (n = 4/6, 67%). Almost all patients (n = 27/28, 96%) reported that DM affected their hobbies, leisure activities, and/or social life in some way. Patients reported difficulty socializing due to DM symptoms (n = 16/28, 57%), and just under half of patients (n = 12/28, 43%) reported that their work was impacted. Five patients (n = 5/28, 18%) reported being unable to work because of DM previously or at the time of the interview. Four patients (n = 4/28, 14%) reported they had difficulty working because of DM, as their symptoms caused issues with pain when wearing their work equipment and caused challenges when lifting items at work. Lastly, one-third of patients (n = 9/28) reported a negative impact on sleep.
Conceptual Model of DM and Assessment Conceptual Saturation
The draft conceptual model developed from the literature was further refined using concepts elicited from the patient interviews (Fig. 2). The topline model provides a visual representation of DM in adult patients. The model assumes three main groups of symptoms: skin, muscle/joint, and other. Some symptoms, such as pain, are common between the groups. The arrow at the top of the conceptual model details how the severity and frequency of symptoms can fluctuate, often referred to by patients as ‘disease flares.’ As the impacts were experienced because of the symptoms of DM, they are presented beneath the symptom boxes and connected by an arrow. The impacts are presented at the domain level within the topline model because of the study being focused on the identification of DM signs/symptoms. However, further details on the impact concepts elicited are presented in the supplementary material (Table S4), and a more in-depth conceptual model with impact details is provided in the Supplementary materials (Figure S3).
Conceptual saturation was assessed by splitting the sample into four equal-sized groups of patients (n = 7/28 per group). Despite a small number of concepts being identified from the last group (n = 5 concepts, all symptoms: muscle numbness, hair loss, weight gain, headaches, stomach upset), none were deemed as disease defining to the patient experience of DM, and all were reported by one patient only.
Discussion
Qualitative interviews with patients confirmed that the overall presentation of DM manifestations is highly variable. The results of this study are consistent with a recent review conducted by DeWane et al. [28]. The most common patient-reported skin symptoms were rash, itch, and dry skin. Patient reports of skin symptoms varied greatly in terms of appearance (across body areas), severity (during and after a flare), and frequency (regularity of flares). Some symptoms (e.g., itch) may be challenging for clinicians to assess and would require a fit-for-purpose PRO measure, while some reported signs, such as erythematous (red) rash, may be better suited for a clinician-reported outcomes (ClinRO) measure. Among the patients that experienced rash, there was a high degree of variation in presentation and in the descriptors used for its appearance, feeling, and texture. Descriptions of rash appeared to be influenced by patient skin tone, and a small number of patients commented that this may make it harder for clinicians to assess their rash accurately. Despite this, descriptions of rash given by Caucasian and non-Caucasian patients were largely aligned, with most describing their rash as red, bumpy, and dry. The head/face and hands were most affected by cutaneous manifestations. Additionally, patients felt that the head/face and hands were the most bothersome and important-to-treat body areas, attributed to the visibility of these areas, and the associated psychological and social impact. All patients with muscle manifestations experienced muscle weakness, and many also reported muscle/joint pain and joint mobility issues. These symptoms led to a wide variety of associated impacts but mostly feeling frustrated by their inability to complete activities at home and work.
We acknowledge some limitations of this study. Qualitative data interpretation can be influenced by the interviewers’ and analysts’ personal biases. This was mitigated by limiting the number of interviewers to two, which kept any interviewer effects consistent and to a minimum. Additionally, although the sample of patients with DM was demographically diverse, all patients were based in the US. There were also limited data collected surrounding the stage of the disease, which could affect patients’ experiences with and emotions towards different signs/symptoms. As there was a vast range of time since diagnosis (0.2–23 years, see Table 1), patients were likely at different points of their disease journey. Therefore, caution should be taken when applying these findings to other geographies without further investigation. It was also not possible to recruit the required quotas set for gender, with more females than males interviewed, although this reflects the prevalence of DM reported in the studies identified in the literature search. Although saturation of core, proximal symptoms associated with skin manifestation of DM was reached, a small number of new concepts arose in the final set of interviews. Despite saturation not being reached against the formal definitions within the literature, the researchers felt they had obtained and reported a broad and deep understanding of DM. It is important to note that saturation is not just meeting a number but also obtaining a full understanding of the research question or indication [29]. As DM is such a variable and heterogeneous condition, it would be expected that many new, individualized concepts may arise, as noted with other heterogeneous diseases [30]. It is known that several distinct clinical phenotypes of DM exist which are clinically categorized by the presence of certain auto-antibodies, such as those against anti-transcriptional intermediary factor 1-γ (TIF1-γ), Mi-2, SUMO-activating enzyme subunit 1/2 (SAE1/2), anti-nuclear matrix protein 2 (NXP-2), and melanoma differentiation-associated protein 5 (MDA-5) [31]. In this study, the distinct clinical phenotype/auto-antibody present for each patient was not collected; therefore, it is possible that certain phenotypes were under- or over-sampled.
The paucity of high quality, qualitative data in adult DM makes comparisons to the existing literature challenging. This qualitative study obtained patient perceptions of their DM experience, which may aid in improving patient experiences by ensuring that relevant COA measures are included in pivotal trials of new therapies. The findings align with previous studies, which have highlighted the need to include the patient perspective [32,33,34]. These data can be used to better understand the concepts of priority for measurement in clinical trials and ultimately help select fit-for-purpose COAs for use in DM.
Data Availability
Data available upon request.
References
UK M. Dermatomyositis 2023 [April 2024]; Available from: https://www.myositis.org.uk/myositis-info/conditions/dermatomyositis/.
Robinson AB, Reed AM. Clinical features, pathogenesis and treatment of juvenile and adult dermatomyositis. Nat Rev Rheumatol. 2011;7(11):664–75.
Jennifer LHM, Christie LC, Wei L, Beverly S, Gil Y, Steven F, Joseph LJ. Cutaneous symptoms of dermatomyositis significantly impact patients’ quality of life. J Am Acad Dermatol. 2006;54(2):217–20.
Ujiie H, Rosmarin D, Schön MP, Ständer S, Boch K, Metz M, et al. Unmet medical needs in chronic, non-communicable inflammatory skin diseases. Front Med (Lausanne). 2022;9: 875492.
Ujiie H, Rosmarin D, Schoen MP, Staender S, Boch K, Metz M, et al. Unmet medical needs in chronic non-communicable inflammatory skin diseases. Front Med-Lausanne. 2022;9:9.
Ahmed S, Chakka S, Concha J, Krain R, Feng R, Werth VP. Evaluating important change in cutaneous disease activity as an efficacy measure for clinical trials in dermatomyositis. Br J Dermatol. 2020;182(4):949–54.
Castro-Molina SA, Méndez-Flores S. Anti-MDA5 dermatomyositis. Literature review. Rev Med Inst Mex Seguro Soc. 2023;61(1):99–105.
Findlay AR, Goyal NA, Mozaffar T. An overview of polymyositis and dermatomyositis. Muscle Nerve. 2015;51(5):638–56.
The Myositis Association. Dermatomyositis. Available at: https://www.myositis.org/about-myositis/types-of-myositis/dermatomyositis/ (Accessed July 2023).
Johns Hopkins Medicine. Dermatomyositis. Available at: https://www.hopkinsmedicine.org/health/conditions-and-diseases/dermatomyositis#:~:text=Dermatoyositis%20is%20the%20term%20used,be%20diagnosed%20with%20the%20disease. (Accessed July 2023).
Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med. 2001;134(12):1087–95.
Hu T, Vinik O. Dermatomyositis and malignancy. Can Fam Physician. 2019;65(6):409–11.
Okogbaa J, Batiste L. Dermatomyositis: an acute flare and current treatments. Clin Med Insights Case Rep. 2019;12:1179547619855370.
Olazagasti JM, Baez PJ, Wetter DA, Ernste FC. Cancer risk in dermatomyositis: a meta-analysis of cohort studies. Am J Clin Dermatol. 2015;16(2):89–98.
Bogdanov I, Kazandjieva J, Darlenski R, Tsankov N. Dermatomyositis: current concepts. Clin Dermatol. 2018;36(4):450–8.
Rolfe DE, Ramsden VR, Banner D, Graham ID. Using qualitative Health Research methods to improve patient and public involvement and engagement in research. Res Involv Engag. 2018;4(1):49.
Livermore P, Gray S, Mulligan K, Stinson JN, Wedderburn LR, Gibson F. Being on the juvenile dermatomyositis rollercoaster: a qualitative study. Pediatr Rheumatol Online J. 2019;17(1):30.
Food and Drug Administration (FDA). Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009.
Food and Drug Administration (FDA). Patient-Focused Drug Development: Collecting Comprehensive and Representative Input. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. 2020.
Food and Drug Administration (FDA). Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments. Guidance for Industry (DRAFT), Food and Drug Administration Staff, and Other Stakeholders. 2022.
Food and Drug Administration (FDA). Patient-focused drug development: methods to identify what is important to patients. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. 2022.
Food and Drug Administration (FDA). Patient-Focused Drug Development: Incorporating Clinical Outcome Assessments into Endpoints for Regulatory Decision-Making. Guidance for Industry (DRAFT). 2023.
O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89(9):1245–51.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59–82.
Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. Qual Rep. 2015;20(9):1408–16.
Kronzer VL, Kimbrough BA, Crowson CS, Davis JM III, Holmqvist M, Ernste FC. Incidence, prevalence, and mortality of dermatomyositis: a population-based cohort study. Arthritis Care Res. 2023;75(2):348–55.
DeWane ME, Waldman R, Lu J. Dermatomyositis: clinical features and pathogenesis. J Am Acad Dermatol. 2020;82(2):267–81.
Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: A systematic review of empirical tests. Soc Sci Med. 2022;292: 114523.
Degboe A, Knight SL, Halling K, Trigg A, Al-Zubeidi T, Aldhouse N, et al. Patients’ experience of recurrent/metastatic head and neck squamous cell carcinoma and their perspective on the EORTC QLQ-C30 and QLQ-H&N35 questionnaires: a qualitative study. J Patient Rep Outcomes. 2017;2:33.
Hodgkinson LM, Wu TT, Fiorentino DF. Dermatomyositis autoantibodies: how can we maximize utility? Ann Transl Med. 2021;9(5):433.
Alexanderson H, Grande MD, Iii COB, Orbai A-M, Sarver C, Clegg-Smith K, et al. Patient-reported outcomes and adult patients’ disease experience in the idiopathic inflammatory myopathies report from the OMERACT 11 myositis special interest group. J Rheumatol. 2014;41(3):581–92.
Christopher-Stine L, Ciesluk A, Chinoy H, Goyal NA, Gunter K, Isenberg D, et al. The dermatomyositis disease symptom questionnaire (DM-DSQ): a measure to assess the patient experience of dermatomyositis symptoms. J Rheumatol
Kleitsch J, Weiner JD, Pandya R, Concha JS, Lim D, Werth VP. The physical and emotional impact of cutaneous dermatomyositis: a qualitative study. Arch Dermatol Res. 2023;315(8):2431–5.
Acknowledgements
The authors would like to acknowledge the significant support and contributions of Myositis Support and Understanding and all of the patients who participated in this research.
Medical Writing and Editorial Assistance
Thank you to Eleanor Ward, Medical Writer, Clarivate. The medical writing and editorial assistance was funded by Pfizer US.
Funding
This work and the journal’s Rapid Service Fee were funded by Pfizer US.
Author information
Authors and Affiliations
Contributions
Stephanie McKee, Jason Xenakis, Harriet Makin, Chris Marshall, Randall Winnette, Rohit Aggarwal, and Sarah Knight all provided input to the concept and design of the study and the drafting of the manuscript. Stephanie McKee, Harriet Makin, Chris Marshall, and Sarah Knight all conducted the interviews and the analysis of this study.
Corresponding authors
Ethics declarations
Conflict of Interest
The study was funded by Pfizer Inc., and Jason Xenakis is an employee of Pfizer Inc. Randall Winnette, who is currently employed by Novartis, was also an employee at Pfizer Inc. when this research was conducted. The authors indicated no further potential conflicts of interest. Randall Winnette is a current employee of Novartis. This research was conducted when Randall Winnette was an employee at Pfizer Inc.
Ethical Approval
Ethical review of the patient interview part of this study was sought from the WCG IRB in the US. Approval date: 8 September 2021, IRB tracking number: 20214484, study number: 1316795. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Written informed consent was obtained from patients prior to their interview, and they were compensated for their time.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
McKee, S., Xenakis, J., Makin, H. et al. Cutaneous Manifestations in Patients with Dermatomyositis, Are They Only Skin Deep?. Dermatol Ther (Heidelb) (2024). https://doi.org/10.1007/s13555-024-01266-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13555-024-01266-1