Abstract
Introduction
The burden of atopic dermatitis (AD) is significant, with a substantial impact on quality of life (QoL). This cross-sectional study aimed to ascertain the burden of AD, its impact on QoL, and associated costs.
Methods
Patients with moderate-to-severe AD were enrolled from eight territories, namely Hong Kong, India, Japan, Mainland China, Singapore, South Korea, Taiwan, and Thailand. After screening was performed and informed consent was obtained, eligible participants were asked to provide responses on their AD symptoms, severity, treatment, and out-of-pocket costs via an online survey. QoL was assessed using EQ-5D-5L and Dermatology Life Quality Index (DLQI), while productivity loss was quantified using the Work Productivity and Activity Impairment (WPAI) questionnaire. Data from completed submissions were analyzed using descriptive statistics. The study was reviewed by the institutional review board in each territory.
Results
Median age of enrolled patients (N = 1103) was 41.0 years (interquartile range, IQR 16.0). The majority of patients reported that their head/neck, trunk, upper limbs, and lower limbs were affected during a flare. Topical (74.2%) and oral steroids (58.7%) were frequently prescribed to manage AD. Common atopic comorbidities were allergic urticaria (64.2%), allergic rhinitis (61.8%), and allergic conjunctivitis (51.5%). Median DLQI score was 13.0 (IQR 11.0), while median EQ-5D-5L (based on China value set) score was 0.8 (IQR 0.4); 87.2% and 77.2% of patients reported pain/discomfort and anxiety/depression on the EQ-5D-5L domains, respectively. Median total annual costs associated with AD were USD 10,128.52 (IQR 12,963.26) per patient, with indirect costs being the largest component. Findings from WPAI indicated that presenteeism is a major contributor to productivity loss.
Conclusion
This multinational survey study showed that AD is associated with substantial QoL impairment and economic burden among Asian adult patients with moderate-to-severe AD. To alleviate burden of AD, clinicians should be more proactive in managing other concomitant conditions including psychological issues, and advocate for increased reimbursement for AD treatments.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
A multinational cross-sectional survey was conducted in Asian adult patients with moderate-to-severe AD to assess the burden of atopic dermatitis (AD), its impact on quality of life (QoL), and associated costs. |
AD was found to have a substantial impact on QoL, with the majority of patients experiencing pain/discomfort, as well as anxiety/depression. |
Most patients were also affected financially to some or great extent due to AD, with median annual total costs incurred per patient estimated to be USD 10,128.52. |
Indirect costs were the largest component of total costs, followed by direct medical costs and direct non-medical costs. |
Productivity loss in patients with AD can largely be attributed to presenteeism. |
Introduction
Atopic dermatitis (AD) is a common chronic and relapsing inflammatory skin disease that affects people of all ages [1]. The pathogenesis of AD is multifactorial, involving various skin lesion types caused by the complex interplay of factors such as impaired skin barrier function, immune dysregulation, genetic susceptibility, and environmental factors [1, 2]. AD has been primarily considered as a childhood disease, affecting 10–30% of children in developed countries [3]. However, it also affects a significant number of adults. Adults could experience persistent AD from childhood, adult-onset AD or recurrent AD, with a lifetime prevalence as high as 20% [3,4,5,6]. Up to 10% of adults in developed countries grapple with this disease, while developing countries are witnessing a steady rise in its prevalence [3, 7]. In some countries such as Singapore and Japan, the prevalence of AD in adults is reported to be higher (approximately 11–13%) as compared to other countries such as China and Korea, likely due to the higher rates of disease onset in adulthood [7, 8].
AD is characterized by intense pruritis and inflammation. It is often associated with sleep disturbance, depression, and anxiety and hence has the potential to drastically affect one’s well-being [1, 9]. Other clinical signs of AD include erythema, xerosis, erosions/excoriations, oozing, and lichenification. In addition, patients may have concomitant atopic (e.g., asthma, food allergy) and/or non-atopic comorbidities (e.g., anxiety, depression) [2]. These comorbidities are often intertwined with AD, contributing to the overall disease burden [2]. The self-stigmatization surrounding chronic skin disease such as insecurities with one’s appearance and feeling of shame could also result in negative social interactions, hinder intimate relationships, and in turn have a substantial psychosocial impact on patients [10, 11]. All of these may significantly impair the quality of life (QoL) of affected patients, particularly for those with more severe AD.
For many patients with AD, whether disease onset occurs during childhood or adulthood, not all cases of AD remain mild as in the early stages of disease. Often, the disease gradually worsens into more severe AD. As AD is a chronic inflammatory disease often accompanied by frequent flares, long-term self-care in daily life is necessary. As a result of the multifactorial aspect of the disease, treatment is typically prescribed for symptomatic control instead of curing the disease [12]. Patients also often take self-help measures to improve their symptoms, such as avoiding allergens and other aggravating factors, caring for their skin to reduce irritation, wearing special clothing, and following a specific diet. This often results in patients spending a significant amount of money on a diverse range of methods in their attempt to alleviate AD over an extended period of time.
Several papers have been published that examine the burden of AD in Japan. One of them presented QoL, work productivity, activity impairment, and healthcare resource utilization data from the 2013 National Health and Wellness Survey [13], while another estimated cost-of-illness using a web-based survey [14]. There are also other similar studies conducted in Western countries [15, 16]. However, few studies have reported QoL and economic burden of adult patients with AD in other territories within the Asia–Pacific region. Hence, this multinational study aimed to ascertain the burden of moderate-to-severe AD, its impact on QoL, and associated costs from the perspective of patients in Asia.
Methods
Study Design and Participants
This was a non-interventional, cross-sectional, self-administered survey study. Patients with AD were enrolled from March to August 2023 in eight territories, namely Hong Kong, India, Japan, Mainland China, Singapore, South Korea, Taiwan, and Thailand.
A steering committee (StC) comprising a dermatology expert from each territory, totalling eight members, was formed as part of the research team. The StC played a critical role in refining the study design and ensuring face validity of our questionnaire. The survey initially had 49 questions in English. It was revised with feedback from the StC and translated into local languages by a professional agency. The institutional review board (IRB) in each territory reviewed the translated materials. Ethical approval was received from the following IRBs: Korea University Ansan Hospital, Chulalongkorn University, Father Muller Hospital, NPO MINS, National Taiwan University Hospital, Hong Kong Doctor Union and Sir Run Run Shaw Hospital. The Parkway Independent Ethics Committee granted an exemption from review. The study was conducted in accordance with the protocol, Guidelines for Good Pharmacoepidemiology Practices, and the Declaration of Helsinki. After IRB approval was obtained, the local language version of the survey was piloted among two patients in each territory to ensure that there were no issues with language and survey programming. Data collected during the pilot phase were included in the final analysis.
An online screener was sent to registered members of a commercial panel. Eligible participants were invited to participate in the online survey if they met the inclusion and exclusion criteria. The inclusion criteria included (1) legal adult age in the respective territory, (2) diagnosis of AD by a physician (self-reported), (3) fulfilled at least three of the Hanifin-Rajka major criteria [17], (4) received medical care for AD symptoms in the past 3 months, and (5) self-rated moderate or severe AD symptoms for last flare in the previous 3 months on the basis of the Patient Global Impression of Severity (PGIS) [18, 19].
Patients with any of the following conditions were excluded: (1) no internet access to participate in the online survey or failure to complete the survey (considered as withdrawal of consent), and (2) those who employed in market research, advertising, healthcare, and pharmaceutical industries (to avoid artificial or biased responses).
All eligible participants provided informed consent electronically. They also received compensation from the commercial panel for time spent completing the survey.
Measurement of AD Severity
The study used the Patient-Oriented Eczema Measure (POEM) to assess participants’ perception of their AD severity over the past week [20]. The POEM questionnaire comprises seven questions. All items are weighted equally and scored on a scale of 0 to 4: no days = 0, 1–2 days = 1, 3–4 days = 2, 5–6 days = 3, every day = 4. The total score of the questionnaire (when scores are summed up across all questions) ranges from 0 to 28 points. Subsequently, patients with AD can be classified into five levels of disease severity based on the total score: 0–2 = clear or almost clear, 3–7 = mild eczema, 8–16 = moderate eczema, 17–24 = severe eczema, and 25–28 = very severe eczema. Missing data were recorded as 0. The use of the POEM questionnaire in this study was registered with the copyright holder [20].
Measurement of QoL
This study measured QoL using the Dermatology Life Quality Index (DLQI) and EQ-5D-5L questionnaires in the respective local languages.
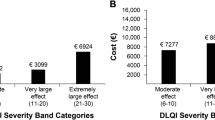
The DLQI consists of 10 questions divided into six aspects, namely symptoms and feelings, daily activities, leisure, work and school, relationships, and treatment [21]. Each question measures the impact of skin disease in the previous week (i.e., last 7 days) and is scored on a scale of 0 to 3, where 0 means no effect at all and 3 indicates high impact. Scores for each item are summed to obtain the final score which ranges from 0 to 30: 0–1 = no effect, 2–5 = small effect, 6–10 = moderate effect, 11–20 = very large effect, and 21–30 = extremely large effect [22]. Higher scores are associated with higher impairment on QoL [21].
The EQ-5D-5L is a generic QoL instrument comprising of a descriptive system and a visual analogue scale (EQ-VAS) [23]. The descriptive system has five dimensions, namely mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has five levels: no problems = 1, slight problems = 2, moderate problems = 3, severe problems = 4, and extreme problems = 5. The scores of all items are combined to form a health state value. In this study, the health state values were converted into utilities by applying value sets from the United Kingdom (UK) and China across all territories. The EQ-VAS records the respondent’s self-rated health from 0 to 100 on a vertical VAS, where 0 represents “the worst health you can imagine” and 100 represents “the best health you can imagine” [23].
Permission to use the DLQI and EQ-5D-5L questionnaires in the study was obtained from the respective copyright holders [23, 24].
Measurement of Loss in Productivity
To assess health-related work productivity loss for the employed population, the Work Productivity and Activity Impairment (WPAI) questionnaire was used. In this study, all participants working full- or part-time were invited to complete the WPAI questionnaire. WPAI outcomes are expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity [25]. Scores input by the study’s participants were calculated with reference to Chan et al.’s method for absenteeism, presenteeism, overall work impairment, and activity impairment [26].
Measurement of Economic Burden
Direct medical costs, direct non-medical costs, and indirect costs associated with AD were analyzed in local currencies and later converted to United States (US) dollars based on the average exchange rates across 2022 (Table S1, Supplementary Materials). The annual total costs were calculated by summing up the annual direct medical costs, annual direct non-medical costs, and annual indirect costs.
Direct medical and non-medical costs paid out-of-pocket were estimated using a bottom-up approach for primary data collection. The direct medical costs was calculated by adding up the costs for all visits to doctor, hospitalizations, traditional medicines, and non-prescription health products in the past quarter (3 months). The direct non-medical costs were calculated by multiplying the transport cost per return trip by the number of doctor visits in the previous quarter. The quarterly cost was then multiplied by 4 to obtain the annual costs over 12 months.
The indirect costs were estimated using the human capital approach. This is relevant for those who are productive (in the formal workforce), but not for people who are unable to work (retired or ill health) or choose not to work. The loss of productivity was calculated using average work hours per week multiplied by the percentage of work impairment. The annual indirect costs was subsequently calculated using productivity loss multiplied by the median annual wage reported in each territory’s official government website (Table S2, Supplementary Materials).
Statistical Methods
Results were summarized using descriptive statistics. Continuous variables were analyzed using medians and interquartile ranges (IQRs), while categorical variables were reported as frequencies and proportions. Any observations that were more than ± 1.5 IQR above or below the median were considered outliers [27]. Outliers identified from cost variables were imputed using the median value calculated among participants from the specific territory. However, outliers identified from WPAI were imputed using the median value calculated among all participants. The analyses were performed using statistical software, SAS version 9.4.
Results
A total of 1103 patients with moderate-to-severe AD were included in the analysis (Table S3, Supplementary Materials), and their demographic characteristics are summarized in Table 1. The median age of the participants was 41.0 years (IQR 16.0). The proportion of male patients (51.9%) was marginally higher than that of female patients (48.1%). In addition, the majority of patients had completed their undergraduate degree (54.6%) and were employed (92.1%) at the time of survey participation.
Clinical Characteristics
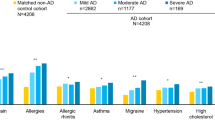
The reported median duration of their last flare was 7.0 days (IQR 6.0) (Table 2). More than half of the patients reported that their head/neck (50.3%), trunk (58.7%), upper limbs (65.7%), and lower limbs (61.9%) were affected during a flare in the past 3 months. The median POEM score was 12.0 (IQR 9.0), and 78.0% of recruited patients were classified as having moderate to very severe eczema. The common therapies used in the last 3 months to manage AD were topical steroids (74.2%), followed by oral steroids (58.7%). The top three most common atopic comorbidities reported were allergic urticaria (64.2%), allergic rhinitis (61.8%), and allergic conjunctivitis (51.5%).
Impact on QoL
Overall, the median DLQI score was 13.0 (IQR 11.0), with 65.5% of patients reporting that AD had a “very large” or “extremely large” effect on their life (Table 3). The median EQ-5D-5L scores were 0.8 (IQR 0.4) using the China value set, and 0.8 (IQR 0.3) using the UK value set. The median EQ-VAS score was 64.0 (IQR 30.0). The majority of patients reported “no problem” in mobility (59.9%) and self-care (60.3%), while 48.3% of patients had no problem with their usual activities (Fig. 1). There were also relatively higher proportions of patients who reported slight to extreme problems in components of pain/discomfort (87.2%) and anxiety/depression (77.2%), when compared to other response options.
Economic Burden
The annual costs per employed patient with AD are summarized in Table 4. Overall, the reported median annual direct medical costs, direct non-medical costs, and indirect costs were USD 915.93 (IQR 2023.57), USD 138.58 (IQR 523.24), and USD 6655.68 (IQR 11,342.90), respectively. The annual costs data for each cost component and territory are shown in Table S4 (Supplementary Materials). The median number of physician visits was 4.0 (IQR 5.0), and median cost of visits was USD 108.90 (IQR 257.21) per patient in the past 3 months (Table S5, Supplementary Materials). Apart from therapies prescribed by physicians, some patients with AD also used traditional medicines and non-prescription health products. In the past quarter, the median costs spent on these were USD 38.09 (IQR 148.79) and USD 51.08 (IQR 129.92) per patient, respectively. The median cost of transportation (round trip) spent by each patient for a physician’s visit was USD 8.84 (IQR 24.79).
Work productivity and activity impairment of patients with AD are summarized in Table S6 (Supplementary Materials). The median percentages of absenteeism and presenteeism were 9.1% (IQR 21.4%) and 60.0% (IQR 40.0%), respectively. When absenteeism and presenteeism were both considered, the median percentage of overall work impairment was 61.5% (IQR 37.8%). The median percentage of activity impairment was 60.0% (IQR 30.0%), whereby patients’ regular daily activities were restricted as a result of AD. Overall, median total annual costs associated with AD were USD 10,128.52 (IQR 12,963.26) per patient (Table 4). The majority of patients (73.5%) mentioned that AD caused a financial burden to some or a great extent.
Discussion
In this study, we conducted an online survey among patients with moderate-to-severe AD across eight territories to identify their characteristics, as well as to estimate the burden of AD, its impact on QoL, and associated costs. The median duration of a flare was reported to be 7.0 days, and most patients were familiar with the use of topical or oral steroids to manage their condition. AD was found to have a considerable impact on patients’ QoL, particularly in domains of pain/discomfort and anxiety/depression. The majority of patients perceived that they are affected financially to some or great extent due to AD. In addition, AD resulted in a median annual total cost of USD 10,128.52 per patient, with a large proportion comprising indirect costs (loss of productivity).
Participants in our study were evaluated on their AD severity using two instruments. During screening, participants used the PGIS tool to self-report the severity of AD symptoms experienced on the basis of their last flare in the previous 3 months. Those who reported moderate or severe symptoms were eligible to continue with the study. Subsequently, the POEM questionnaire was administered to all eligible patients. Our results showed that on the basis of the POEM scoring, 19.4% of patients were considered to have mild eczema, while 2.6% had clear or almost clear skin. This is likely due to the design of the POEM questionnaire which requires participants to recall and answer questions on their AD symptoms over a shorter time period of 1 week, instead of 3 months. These patients could have recovered from their last AD flare or were in remission at time of survey participation. This discrepancy in severity assessment findings underscores the dynamic and time-sensitive nature of AD, and should be considered when interpreting our results.
Our study revealed that over half of the participants experienced a flare in the past 3 months, primarily affecting areas such as the upper limbs, lower limbs, trunk, and head/neck. This aligns with previous research indicating that the head and neck are common areas affected by AD [28]. Notably, a study in China also found that the antecubital fossae, followed closely by the neck, were the most frequently affected areas [29]. Certain body parts may take longer to heal and respond to treatment as a result of factors including skin thickness and likelihood of exposure to chemical products or external irritants [30]. Further research is warranted to establish a possible link between the affected body part and duration or severity of flares among patients with AD. This insight could offer valuable guidance for treatment approaches based on the body part affected.
Topical steroids were reported to be the most commonly used treatment for AD among our study’s participants. This observation aligns with expected prescribing practice, as topical steroids are recommended as an induction therapy in both Asian and international AD clinical guidelines [31,32,33,34,35,36,37]. The frequency of topical steroid application should be reduced when there are signs of inflammation subsiding [34]. Along with topical steroids, a moisturizer or emollient can be used in conjunction as it prevents dry skin, restores the barrier function of the epidermis, and reduces the likelihood of flares [35, 38, 39]. The second most common treatment used by participants was oral steroids. They are not recommended to be used routinely by guidelines, as prolonged usage can lead to severe systemic adverse effects [31, 33]. However, a short course of oral steroids may be useful to treat severe AD symptoms during acute flares [31, 33]. Given that this study enrolled patients with moderate-to-severe AD, and a significant proportion of them were contending with ongoing severe or very severe eczema, the reported use of oral steroids for rapid relief may not be surprising. However, it is necessary to examine whether oral steroids are used frequently or for long periods of time in such severe patients. Apart from steroids, other therapies that were more frequently reported in our study include immunosuppressants (e.g., cyclosporin, methotrexate, azathioprine), injectable dupilumab and oral JAK inhibitors (e.g., upadacitinib, baricitinib, abrocitinib). These treatments are usually prescribed for moderate-to-severe AD [40, 41]. The availability of newly developed drugs may differ among countries, and a review of their use in each country, including oral steroids, is needed.
Our study results showed that most patients with AD had atopic comorbidities such as allergic rhinitis and allergic urticaria. This finding is consistent with several previous studies. Allergic rhinitis is highly prevalent among patients with AD, and is part of a few diagnostic criteria (such as Hanifin and Rajka and UK Working Party’s Diagnostic Criteria for Atopic Dermatitis) used in AD [42,43,44]. Among Danish patients with AD, it was also observed that AD is significantly related to chronic urticaria [45]. In addition, the perception of food allergies as an atopic comorbidity among patients in our study is consistent with the significant association between AD severity and food allergies reported by a study in the USA [46]. The presence of atopic comorbidities alongside AD compounds the burden on patients, necessitating treatments not only for AD but also for these concomitant conditions. According to an earlier study, over half of the participants with AD required two or more prescribed therapies to effectively manage their comorbidities [47]. Hence, to improve overall clinical outcomes, it is important for physicians to be aware of the presence of atopic comorbidities and holistically manage them.
Our study used the DLQI questionnaire to assess the impact of AD on QoL. The findings from DLQI indicated that about two-thirds of patients felt that AD has a “very large effect” or “extremely large effect” on their lives. In a recent systematic review conducted to understand the QoL of Asians with AD, most included studies suggested that AD has a substantial impact (“moderate effect” or “very large effect”) on patients’ QoL [48]. Our study might have shown a greater impact of AD on QoL as we included participants with moderate-to-severe AD, while the systematic review did not exclude studies that encompassed patients with mild AD. Our study results align more closely with another study conducted in India, where the majority of participants had moderate and severe AD. More than half of the participants reported that AD has a “very large effect” and “extremely large effect” on their lives [49]. Therefore, we can infer that the severity of disease does affect the degree of QoL impairment in patients with AD.
To ensure better comparability of our EQ-5D-5L results with other studies within or outside of Asia, we separately used value sets for China and UK when converting health state values obtained from each territory into utilities. There was little difference in our results, despite the use of two value sets. Our study’s median EQ-5D-5L score (0.8) was slightly lower, compared with a Japanese study’s median EQ-5D-5L score (0.9) among patients with AD [50]. Furthermore, an AD disease registry in Japan which included adults with moderate-to-severe AD reported a median EQ-5D-5L score of 0.8 and a median EQ-VAS score of 70 [51]. In a Hungarian study and the National Health and Wellness Survey (consisting of patients from the United States of America, UK, France, and Germany), the reported median EQ-5D-5L scores were 0.9 and 0.8, respectively [52, 53]. These are similar to our study’s findings. In addition, higher proportions of our participants experienced problems in the pain/discomfort and anxiety/depression domains, in contrast to the other domains within EQ-5D-5L. This finding is in agreement with results from another study which employed the Skindex-16 instrument, indicating that the emotional impact of AD tends to outweigh its functional impairments in patients [48]. The pain/discomfort experienced may be caused by AD symptoms such as pruritus, soreness, painful wounds, and stinging skin [54]. It is also not uncommon for patients to face issues related to anxiety/depression. This can be due to various factors, including the adverse impact of AD on a patient’s sleep duration and quality [55]. The physical skin appearance of patients with AD can lead to social stigma or discrimination from others who may mistakenly believe that AD is contagious [11]. Such misconception is likely to cause people to avoid those suffering from AD, leading to decreased social interaction. The resulting isolation could contribute to lower confidence, lower self-esteem, and heightened feelings of embarrassment, potentially leading to psychological problems in patients [56].
The majority of our study participants expressed that AD imposes a financial burden to some or a great extent. When the different cost components were assessed, indirect costs constituted the largest proportion of total costs, followed by direct medical costs and direct non-medical costs. This pattern has also been reported in various other European studies, as well as in a systematic review [4, 15, 57]. On the basis of findings from a study in Taiwan, productivity loss similarly constituted the most substantial portion of the economic burden experienced by individuals with moderate and severe AD [58]. The mean costs of productivity loss among patients with severe AD (USD 9310.17, using the conversion rate from the study) closely resemble the median costs of productivity loss among the Taiwanese participants in our study (USD 9391.20) [58]. Sleep disturbance among employed patients with AD can lead to decrease in productivity, stemming from difficulties in concentration and feelings of lethargy [4]. This may explain the notably higher levels of presenteeism (compared with absenteeism), ultimately having a greater impact on indirect costs [4]. When examining the total costs of AD across various territories in our study, we observed that the costs were highest in Singapore (USD 32,158.75) and lowest in Thailand (USD 4851.72). This finding may be attributed to factors such as the availability and reimbursement of AD treatments within each territory which will affect out-of-pocket expenses. However, any comparison of costs should be performed with caution due to differences in healthcare financing system, income level, cost of living, and purchasing power between territories.
This study has several limitations. Firstly, the data relies on self-reported information (e.g., AD diagnosis, symptoms, and severity) gathered through survey, which may be subjective in nature. Secondly, there could be underrepresentation of certain patient groups in our study. The participants were registered with a commercial panel that offers paid online surveys to its members. It is plausible that they are more internet-savvy, younger, and better educated compared with the general AD population. Hence, this may reduce the generalizability of our findings. Lastly, there is a potential for recall bias. Efforts were made to mitigate this by limiting the recall period to 3 months for cost-related variables. However, we cannot exclude the possibility that some patients may still report inaccurate or incomplete costs.
Conclusion
This multinational survey study demonstrated that AD is associated with substantial QoL impairment and economic burden in Asia. Among those with moderate-to-severe AD, QoL was adversely impacted with the majority of patients experiencing issues with pain/discomfort and anxiety/depression. The median annual total cost incurred per patient was estimated to be USD 10,128.52, and most also reported that the financial burden associated with AD had affected them to some or great extent. Although costs may vary considerably across territories, indirect costs were consistently the largest component of total costs. Furthermore, presenteeism was found to be a major contributor to the overall loss in productivity. To reduce the burden of AD, it is crucial for clinicians to look beyond the skin and take a more proactive approach in diagnosing and treating other concomitant conditions including psychological issues. Future studies can also explore strategies towards improving access to novel effective treatments and reducing costs for patients with AD.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–51.
Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144–51.
Kolb L, Ferrer-Bruker SJ. Atopic dermatitis. StatPearls–NCBI Bookshelf [Internet]. Treasure Island (FL): StatPearls:.
Fasseeh AN, Elezbawy B, Korra N, et al. Burden of atopic dermatitis in adults and adolescents: a systematic literature review. Dermatol Ther. 2022;12(12):2653–68.
Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1.
Mortz C, Andersen K, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70(7):836–45.
Lopez Carrera YI, Al Hammadi A, Huang YH, Llamado LJ, Mahgoub E, Tallman AM. Epidemiology, diagnosis, and treatment of atopic dermatitis in the developing countries of Asia, Africa, Latin America, and the Middle East: a review. Dermatol Ther (Heidelb). 2019;9(4):685–705.
Yong AM-Y, Tay Y-K. Atopic dermatitis: racial and ethnic differences. Dermatol Clin. 2017;35(3):395–402.
Gross PA. Epidemiology of hospital-acquired pneumonia. Semin Respir Infect. 1987;2(1):2–7.
Heim-Ohmayer P, Freiberger A, Gedik M, et al. The impact of stigmatization of psoriasis, atopic dermatitis and mastocytosis in different areas of life—a qualitative interview study. Skin Health Dis. 2022;2(4):e62.
Gochnauer H, Valdes-Rodriguez R, Cardwell L, Anolik RB. The psychosocial impact of atopic dermatitis. In: Feldman SR, Strowd LC, Lovell KK, editors. Management of atopic dermatitis: methods and challenges. Cham: Springer; 2017. p. 57–69.
Tang TS, Bieber T, Williams HC. Are the concepts of induction of remission and treatment of subclinical inflammation in atopic dermatitis clinically useful? J Allergy Clin Immunol. 2014;133(6):1615–25.e1.
Arima K, Gupta S, Gadkari A, et al. Burden of atopic dermatitis in Japanese adults: analysis of data from the 2013 National Health and Wellness Survey. J Dermatol. 2018;45(4):390–6.
Murota H, Inoue S, Yoshida K, Ishimoto A. Cost of illness study for adult atopic dermatitis in Japan: a cross-sectional Web-based survey. J Dermatol. 2020;47(7):689–98.
Girolomoni G, Luger T, Nosbaum A, et al. The economic and psychosocial comorbidity burden among adults with moderate-to-severe atopic dermatitis in Europe: analysis of a cross-sectional survey. Dermatol Ther (Heidelb). 2021;11(1):117–30.
Eckert L, Gupta S, Gadkari A, Mahajan P, Gelfand JM. Burden of illness in adults with atopic dermatitis: analysis of National Health and Wellness Survey data from France, Germany, Italy, Spain, and the United Kingdom. J Am Acad Dermatol. 2019;81(1):187–95.
Rothe MJ, Grant-Kels JM. Diagnostic criteria for atopic dermatitis. Lancet. 1996;348(9030):769–70.
Newton L, DeLozier AM, Griffiths PC, et al. Exploring content and psychometric validity of newly developed assessment tools for itch and skin pain in atopic dermatitis. J Patient Rep Outcomes. 2019;3(1):42.
Rhatigan K, Tsami K, Kesavan H, Turner R, Jolley C, Hull J et al. P57 Patient global impression of severity scale characterises symptom severity in chronic cough. Thorax. 2021;76(Suppl 2):A97-A8.
The University of Nottingham. POWM - Patient Oriented Eczema Measure. https://www.nottingham.ac.uk/research/groups/cebd/resources/poem.aspx. Accessed 17 Nov 2023.
Finlay AY, Khan G. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6.
Basra M, Fenech R, Gatt R, Salek M, Finlay AY. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159(5):997–1035.
EQ-5D EuroQol. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Accessed 17 Nov 2023.
Dermatology Life Quality Index Cardiff University. https://www.cardiff.ac.uk/medicine/resources/quality-of-life-questionnaires/dermatology-life-quality-index. Accessed 17 Nov 2023.
WPAI Scoring Reilly Associates. http://www.reillyassociates.net/WPAI_Scoring.html. Accessed 17 Nov 2023.
Chan TC, Lin YC, Cho YT, Tang CH, Chu CY. Impact of Atopic Dermatitis on Work and Activity Impairment in Taiwan. Acta Derm Venereol. 2021;101(9):adv00556.
BOXPLOT Statement SAS Help Center. https://documentation.sas.com/doc/en/pgmsascdc/9.4_3.5/grstatgraph/p0vuh82v39fsasn1vqhzmhdl8y16.htm. Accessed 17 Nov 2023.
Chaplin S. Guide to treatments used for atopic dermatitis in adults. Prescriber. 2016;27(10):30–9.
Wang X, Shi XD, Li LF, Zhou P, Shen YW, Song QK. Prevalence and clinical features of adult atopic dermatitis in tertiary hospitals of China. Medicine (Baltimore). 2017;96(11): e6317.
Upham B. 10 things to know about eczema (atopic dermatitis) on the face and neck October 2022. https://www.everydayhealth.com/eczema/things-to-know-about-eczema-on-the-face-and-neck/. Accessed 17 Nov 2023.
Katoh N, Ohya Y, Ikeda M, et al. Japanese guidelines for atopic dermatitis 2020. Allergol Int. 2020;69(3):356–69.
Tay YK, Chan YC, Chandran NS, et al. Guidelines for the management of atopic dermatitis in Singapore. Ann Acad Med Singap. 2016;45(10):439–50.
Yao T-C, Wang I-J, Sun H-L, et al. Taiwan guidelines for the diagnosis and management of pediatric atopic dermatitis: consensus statement of the Taiwan Academy of Pediatric Allergy, Asthma and Immunology. J Microbiol Immunol Infect. 2022;55(4):561–72.
Kim JE, Kim HJ, Lew BL, et al. Consensus Guidelines for the Treatment of Atopic Dermatitis in Korea (Part I): general management and topical treatment. Ann Dermatol. 2015;27(5):563–77.
Rubel D, Thirumoorthy T, Soebaryo RW, et al. Consensus guidelines for the management of atopic dermatitis: an Asia–Pacific perspective. J Dermatol. 2013;40(3):160–71.
Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26(8):1045–60.
Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26(9):1176–93.
LePoidevin LM, Lee DE, Shi VY. A comparison of international management guidelines for atopic dermatitis. Pediatr Dermatol. 2019;36(1):36–65.
Katoh N, Ohya Y, Ikeda M, et al. Clinical practice guidelines for the management of atopic dermatitis 2018. J Dermatol. 2019;46(12):1053–101.
Kraft M, Worm M. Dupilumab in the treatment of moderate-to-severe atopic dermatitis. Expert Rev Clin Immunol. 2017;13(4):301–10.
Prescription Oral: National Eczema Association; 2023. https://nationaleczema.org/eczema/treatment/immunosuppressants/#:~:text=Immunosuppressants%20are%20prescribed%20for%20moderate,the%20risk%20of%20skin%20infection. Accessed 17 Nov 2023.
Davis DM, Drucker AM, Alikhan A, et al. American Academy of Dermatology Guidelines: awareness of comorbidities associated with atopic dermatitis in adults. J Am Acad Dermatol. 2022;86(6):1335–6.e18.
Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60(92):44–7.
Williams H, Jburney P, Pembroke A, Hay R, Party ADDCW. The UK Working Party’s diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131(3):406–16.
Andersen YM, Egeberg A, Gislason GH, Skov L, Thyssen JP. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76(2):274–80e1.
Silverberg JI, Gelfand JM, Margolis DJ, et al. Association of atopic dermatitis with allergic, autoimmune, and cardiovascular comorbidities in US adults. Ann Allergy Asthma Immunol. 2018;121(5):604–12e.3.
Loiselle AR, Thibau IJ, Johnson JK, Guadalupe M, Begolka WS. Financial and treatment access burden associated with atopic dermatitis comorbidities. Ann Allergy Asthma Immunol. 2024;132(2):243–5.
Huang J, Choo YJ, Smith HE, Apfelbacher C. Quality of life in atopic dermatitis in Asian countries: a systematic review. Arch Dermatol Res. 2022;314(5):445–62.
Nagata A, Kazi T, Akter Z, et al. The influence of atopic dermatitis on health-related quality of life in Bangladesh. Int J Environ Res Public Health. 2021;18(21):11593.
Kamei K, Hirose T, Yoshii N, Tanaka A. Burden of illness, medication adherence, and unmet medical needs in Japanese patients with atopic dermatitis: a retrospective analysis of a cross-sectional questionnaire survey. J Dermatol. 2021;48(10):1491–8.
Katoh N, Saeki H, Kataoka Y, et al. Atopic dermatitis disease registry in Japanese adult patients with moderate to severe atopic dermatitis (ADDRESS-J): baseline characteristics, treatment history and disease burden. J Dermatol. 2019;46(4):290–300.
Koszorú K, Hajdu K, Brodszky V, et al. General and skin-specific health-related quality of life in patients with atopic dermatitis before and during the COVID-19 pandemic. Dermatitis. 2022;33(6S1):S92–103.
Vilsbøll AW, Kragh N, Hahn-Pedersen J, Jensen CE. Mapping Dermatology Life Quality Index (DLQI) scores to EQ-5D utility scores using data of patients with atopic dermatitis from the National Health and Wellness Study. Qual Life Res. 2020;29:2529–39.
Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59(4):e75–91.
Lee SH, Lee SH, Lee SY, Lee B, Lee S-H, Park YL. Psychological health status and health-related quality of life in adults with atopic dermatitis: a nationwide cross-sectional study in South Korea. Acta Derm Venereol. 2018;98(1):89–97.
Kwak Y, Kim Y. Health-related quality of life and mental health of adults with atopic dermatitis. Arch Psychiatr Nurs. 2017;31(5):516–21.
Beretzky Z, Koszorú K, Rencz F, et al. Societal costs and health related quality of life in adult atopic dermatitis. BMC Health Serv Res. 2023;23(1):859.
Lee EM, Cho YT, Chan TC, Shen D, Chu CY, Tang CH. Economic burden of atopic dermatitis in Taiwan. Acta Derm Venereol. 2023;103:adv00866.
Acknowledgements
The authors would like to thank Dr James Wee from Pfizer and Chee Wen Eng from IQVIA for their contribution in managing this project (at time of data collection), and Xiaoyun Chen from IQVIA for performing the statistical analysis. In addition, the authors would like to extend our gratitude to the participants of the study.
Funding
This study (including publication fees) was funded by Pfizer Hong Kong Corporation Limited. IQVIA was contracted to conduct study activities. The sponsor was also involved in the study design, data interpretation and manuscript review.
Author information
Authors and Affiliations
Contributions
Chia-Yu Chu, Yung Chan, Siriwan Wananukul, Hao Cheng, Nisha Suyien Chandran, Ramesh Bhat, Sang Wook Son, Han-Fang Liao, Sean Gardiner, Qi Qing Ng, See-Hwee Yeo, Sophie Bozhi Chen and Yoko Kataoka were involved in the conception and design of this study, interpretation of the results, and critical revision of the manuscript. Qi Qing Ng and See-Hwee Yeo were involved in data analysis and drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Han-Fang Liao and Sean Gardiner are employees of Pfizer. Yoko Kataoka has received lecturer honoraria from Sanofi, AbbVie, Pfizer and Maruho, and research funding from Sanofi, Leo Pharma, Pfizer, Maruho, Lilly, AbbVie, Otsuka, Taiho and Amgen. Yung Chan is a speaker for AbbVie, Bioderma, Galderma, Inova, Eli Lily, Lumenis, Menarini and Pfizer; an advisory board member for AbbVie, Bayer, Bioderma, CeraVe, Eli Lilly, Menarini, Pfizer, Sanofi, Quanta System and Janssen. Chia-Yu Chu is an investigator for AbbVie, Amgen, Dermira, Eli Lilly, Janssen, Novartis, Oneness Biotech, Pfizer, Regeneron Pharmaceuticals Inc., Roche and Sanofi; a consultant for AbbVie, Amgen, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer, Roche and Sanofi; a speaker for AbbVie, Eli Lilly, GlaxoSmithKline, Janssen, Mylan, Novartis, Pfizer, Roche, Sanofi and Viatris; and an advisory board member for AbbVie, Amgen, Mylan, Pfizer, Roche, Sanofi and Viatris. Nisha Suyien Chandran has received fees for participation in advisory boards from AbbVie, Johnson & Johnson, Sanofi, Pfizer, DKSH, L’Oreal and Novartis; investigator fees for clinical trials from AbbVie, Novartis, Amgen, Sanofi and Boehringer Ingelheim; and speaker honoraria from Galderma, Johnson & Johnson, LEO, Pfizer, Sanofi and Lion Corporation. All other authors have no conflict of interest.
Ethical Approval
Ethical approval was received from the following IRBs: Korea University Ansan Hospital, Chulalongkorn University, Father Muller Hospital, NPO MINS, National Taiwan University Hospital, Hong Kong Doctor Union and Sir Run Run Shaw Hospital. The Parkway Independent Ethics Committee granted an exemption from review. The study was conducted in accordance with the protocol, Guidelines for Good Pharmacoepidemiology Practices, and the Declaration of Helsinki. All participants provided informed consent.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chu, CY., Chan, Y., Wananukul, S. et al. Quality of Life and Burden of Moderate-to-Severe Atopic Dermatitis in Adult Patients Within the Asia–Pacific Region: A Cross-sectional Survey. Dermatol Ther (Heidelb) (2024). https://doi.org/10.1007/s13555-024-01244-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13555-024-01244-7