Abstract
Introduction
Treatments for atopic dermatitis (AD) often fail to achieve lasting disease control. In the CrisADe CONTROL phase III study (ClinicalTrials.gov: NCT04040192), participants aged ≥ 3 months with mild to moderate AD treated with once-daily (QD) crisaborole, following initial treatment success with crisaborole twice daily (BID), had longer periods of flare-free maintenance, a higher number of flare-free days, and a lower number of flares compared with those who received vehicle. The study was an exploratory analysis of data on the maintenance of response per Investigator’s Static Global Assessment (ISGA; ISGA score of 0 [clear] or 1 [almost clear]) during the CrisADe CONTROL study through week 52.
Methods
Exploratory endpoints were the time to ISGA response during the open-label run-in period, and the maintenance of ISGA response and the severity and duration of flares during the double-blind maintenance period. Outcomes were stratified by age (participants aged 3 months to < 12 years and ≥ 12 years) and duration of crisaborole BID treatment (< 4 weeks or ≥ 4 weeks) during the open-label run-in period.
Results
During the open-label run-in period, the median time to ISGA response was 41.5 days. From week 4 to week 52 of the double-blind maintenance period, the proportion of participants who maintained ISGA response was greater with crisaborole versus vehicle, and this difference was statistically significant up to week 36 (P < 0.05). Duration of flare periods during the maintenance period were 54.1 and 54.0 days for the vehicle and crisaborole-treated groups, respectively. Numerically fewer crisaborole-treated participants experienced a flare with an ISGA score of ≥ 2 compared with vehicle-treated participants (64.8% vs. 74.4%, respectively). Findings were comparable across most subgroups.
Conclusions
Adult and pediatric participants with mild to moderate AD at baseline who had achieved responder criteria (treatment success) with crisaborole BID during the run-in period maintained response per ISGA with crisaborole QD during the double-blind maintenance period through week 52.
Trial Registration
ClinicalTrials.gov: NCT04040192.
Plain Language Summary
Atopic dermatitis is a skin disease that causes itchy, red, and dry patches of skin that can affect a person for a long time. Current treatments for atopic dermatitis often fail to keep the symptoms under control. Some creams and ointments applied to the skin (known as topical treatments) can ease the discomfort of atopic dermatitis. Crisaborole is a steroid-free ointment that has been shown to improve symptoms of atopic dermatitis in clinical studies. In a study called the CrisADe CONTROL trial, crisaborole was tested to see if it can keep atopic dermatitis symptoms under control. People who participated in the study were aged 3 months and older and they had mild-to-moderate atopic dermatitis. Participants were asked to use crisaborole on their itchy, red, and dry skin twice daily for 8 weeks. Patients were called “responders” if their symptoms became nearly clear or completely clear based on a doctor’s assessment called the Investigator’s Static Global Assessment, which rates atopic dermatitis between clear to severe. Some responders were asked to use crisaborole once daily for 52 weeks and another group of responders was asked to use a control (an ointment with no medicine) once daily for 52 weeks. Investigators looked at how long the skin remained nearly clear or completely clear during the 52 weeks. Results of this study showed that after initial treatment success with crisaborole twice daily, adult and pediatric participants who had mild-to-moderate atopic dermatitis were able to keep their skin nearly clear or completely clear with crisaborole once daily.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Treatments for atopic dermatitis (AD) often fail to achieve long-term disease control. |
The CrisADe CONTROL trial found that long-term maintenance treatment with crisaborole once daily (QD) resulted in delayed onset of first flare, greater number of flare-free days, and decreased number of flares compared to vehicle in patients with mild to moderate AD who responded to crisaborole twice daily (BID). |
This exploratory analysis evaluated the maintenance of response per Investigator’s Static Global Assessment (ISGA; ISGA score of 0 [clear] or 1 [almost clear]) during the CrisADe CONTROL trial through week 52. |
What was learned from this study? |
Participants achieving responder criteria (treatment success) with crisaborole BID during the run-in period maintained response per ISGA with crisaborole QD during the double-blind maintenance period through week 52. |
Results were comparable among subgroups stratified by age (participants aged 3 months to < 12 years and ≥ 12 years) and duration of crisaborole BID treatment (< 4 weeks or ≥ 4 weeks) during the open-label run-in period. |
Introduction
Atopic dermatitis (AD) is a complex chronic immuno-inflammatory skin disease characterized by dry skin, intense pruritus, and recurrent eczematous lesions [1,2,3]. Topical corticosteroids (TCSs) are traditionally considered the mainstay anti-inflammatory agent for AD flares, [1, 4, 5] although topical calcineurin inhibitors (TCIs) have demonstrated comparable efficacy to mild and moderate strength TCS [6, 7]. TCIs are approved for short-term and noncontinuous treatment in recalcitrant AD, and several guidelines have added TCIs as an alternative to TCSs [1, 4, 5].
Often, current treatments for AD do not achieve long-lasting disease control despite initial improvement [8, 9]. Patients often have a fluctuating clinical course characterized by periods of remission interrupted by acute exacerbations (flares) [9]. Moreover, treatment-related efficacy may vary according to several patient-specific factors, including age. Thus, studies evaluating efficacy outcomes among diverse populations are important to achieve a more targeted therapeutic approach in AD [10].
Crisaborole ointment, 2%, is a nonsteroidal phosphodiesterase 4 (PDE4) inhibitor used for the treatment of mild to moderate AD [11]. Crisaborole has been shown to be effective and well tolerated in treating patients aged ≥ 3 months with mild to moderate AD [12,13,14]. The recent CrisADe CONTROL (ClinicalTrials.gov NCT04040192) trials evaluated the long-term efficacy and safety of crisaborole once daily (QD) versus vehicle as maintenance treatment to reduce the incidence of flares in participants aged ≥ 3 months with mild to moderate AD who previously responded to twice-daily (BID) treatment. Compared with vehicle, treatment with crisaborole QD resulted in a longer median time of flare-free maintenance, a higher mean number of flare-free days, and a lower mean number of flares through 52 weeks [15].
In this exploratory analysis of the CrisADe CONTROL trial, we evaluated the proportion of crisaborole- versus vehicle-treated participants who maintained response per Investigator’s Static Global Assessment (ISGA; ISGA score of 0 [clear] or 1 [almost clear]) during the double-blind maintenance period through week 52. In addition, time to ISGA response during the open-label run-in period as well as the severity and duration of flare during the double-blind maintenance period were evaluated.
Methods
Study Design
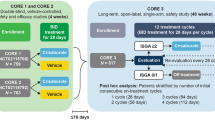
This analysis used data from the randomized, double-blind, vehicle-controlled, 52-week, phase 3 CrisADe CONTROL trial [15]. The final protocol for the CrisADe CONTROL trial and any amendments and informed consent/assent documentation were reviewed and approved by the institutional review boards or institutional ethics committees at each of the investigational centers participating in the study. Details of the study design have been reported previously [15].
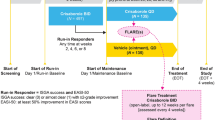
In brief, eligible participants received crisaborole BID during an open-label run-in period of up to 8 weeks. Participants who achieved ISGA success (ISGA score of 0 [clear] or 1 [almost clear] with a ≥ 2-grade improvement from baseline) and ≥ 50% improvement in the Eczema Area and Severity Index (EASI-50) total score from baseline were defined as responders. Responders were randomized to the double-blind maintenance period in a 1:1 ratio to receive either crisaborole or vehicle QD for 52 weeks. Participants were assessed every 4 weeks during the maintenance period for efficacy and safety. If a flare (ISGA score ≥ 2) occurred, the participant entered a flare treatment period, during which the participant was switched from their assigned study treatment to open-label crisaborole BID for up to 12 weeks. If a flare occurred outside of the study visits, participants or caregivers reported the flare directly or by means of an electronic diary; however, the resolution of a flare outside of the scheduled study visits was not reported. Participants were assessed every 4 weeks to ascertain if the flare had resolved (ISGA score ≤ 1), in which case the assigned treatment was resumed [15].
Participants and Treatment
At the time of informed consent/assent, participants were aged ≥ 3 months [15]. Participants had mild to moderate AD, defined as an ISGA score of 2 (mild) or 3 (moderate), as well as a percentage body surface area (%BSA) involved (excluding scalp) of ≥ 5% [15].
Participants and/or their parent(s)/legal guardian(s) were instructed to apply a layer of the study treatment to cover all treatable AD lesions (excluding the scalp), including newly identified lesions during both the open-label period and the double-blind maintenance period. During the open-label run-in period, participants were instructed to apply crisaborole BID throughout 8 weeks, even when the skin became clinically clear. Likewise, throughout the maintenance period, participants were instructed to continue to apply the QD study treatment to the most commonly affected skin areas. Crisaborole was applied BID during all flares occurring during the maintenance period [15].
Endpoints and Assessments
Primary and Key Secondary Endpoints
In the CrisADe CONTROL trial, the primary endpoint was flare-free maintenance until onset of the first flare. Secondary endpoints were number of flare-free days, number of flares, and maintenance of pruritus response until onset of the first flare [15].
Exploratory Assessments
Time to ISGA response was assessed during the open-label run-in period; ISGA response was assessed every 2 weeks. Maintenance of ISGA response (score of 0 [clear] or 1 [almost clear]) was evaluated during the double-blind maintenance period of the study through week 52. Response to study treatment QD per ISGA was assessed during scheduled in-clinic visits every 4 or 8 weeks and by phone at weeks 20, 28, 36, and 44. In the event of a flare during the double-blind maintenance period, the severity and time until flare resolution (ISGA score ≤ 1) was assessed [15]. Participants were assessed every 4 weeks for flare resolution [15]. For this exploratory analysis, endpoints were stratified by age (participants aged 3 months to < 12 years and ≥ 12 years) and duration of crisaborole BID treatment (< 4 weeks or ≥ 4 weeks) during the run-in period.
Statistical Analysis
The overall population consisted of all the participants from the CrisADe CONTROL study who were randomly assigned to a treatment group and met responder criteria. The P-value and estimate of treatment difference for maintenance of ISGA response during the 52-week double-blind maintenance period were calculated from Cochran–Mantel–Haenszel weight method stratified by age group (3 months to < 12 years and ≥ 12 years), duration of the BID treatment in the open-label period (≤ 4 weeks and > 4 weeks), and ISGA score (clear [0], or almost clear [1]) at randomization. The significance level was defined as P = 0.05. For the maintenance of ISGA response during the double-blind maintenance period, the stratified 95% confidence interval (CI) was constructed using the NewCombe method.
Ethical Approval
This planned exploratory analysis of data from the CrisADe CONTROL study was exempt from institutional review board approval. All participants or parents/guardians provided written informed consent for participation in the CrisADe CONTROL study. The CrisADe CONTROL study was approved by the Quorum Review Institutional Review Board and was conducted in accordance with the ethical principles originating in the Declaration of Helsinki.
Results
Baseline Characteristics
A total of 497 participants were included in the open-label run-in period. Overall, 270 participants were enrolled into the 52-week double-blind maintenance period, of whom 135 were randomly assigned to the crisaborole group and 135 were randomly assigned to the vehicle group. However, ten participants in the crisaborole-treated group and six participants in the vehicle-treated group were ultimately excluded from the efficacy analysis because they did not meet responder criteria (ISGA success and EASI-50) at randomization [15].
Demographic and baseline characteristics between the two randomized groups [15] and subgroups (Table 1) were generally balanced; however, participants receiving crisaborole QD following > 4 weeks BID treatment during the open-label period had a numerically greater EASI total score, ISGA score, and %BSA at baseline versus participants who received ≤ 4 weeks BID treatment. Participants aged 3 months to < 12 years treated with crisaborole QD had a numerically greater EASI total score and %BSA at baseline versus participants aged ≥ 12 years treated with crisaborole QD at baseline. Furthermore, participants aged ≥ 12 years had a numerically greater duration of disease than participants aged 3 months to < 12 years. Similarly, participants who had received > 4 weeks of BID treatment during the open-label period had a numerically greater duration of disease than participants who had received ≤ 4 weeks of BID treatment (Table 1).
Primary and Key Secondary Endpoints
Median time of flare-free maintenance (the number of consecutive flare-free days) was longer for patients who received crisaborole versus vehicle (111 vs. 30 days, respectively; P = 0.0034). The mean number of flare-free days (the number of flare-free days throughout the study period) was higher for patients who received crisaborole versus vehicle (234.0 vs. 199.4 days, respectively; P = 0.0346). The mean number of flares was lower for patients who received crisaborole versus vehicle (0.95 vs. 1.36 flares, respectively; P = 0.0042) [15].
Time to ISGA Response During the Open-Label Run-In Period
During the open-label period, the median time to ISGA response was 41.5 days for the overall population. When stratified by age, the median time to ISGA response for participants aged 3 months to < 12 years and those aged ≥ 12 years was 31.0 and 42.0 days, respectively. A numerically greater proportion of participants aged 3 months to < 12 years achieved ISGA response from week 2 to week 6 versus the other age subgroup and the overall population (Fig. 1).
Maintenance of ISGA Response During the Double-Blind Maintenance period
Examples of AD lesions assessed throughout the CrisADe CONTROL trial are shown in Fig. 2. The proportion of participants who maintained ISGA score ≤ 1 (responders) was numerically greater in those treated with crisaborole versus those who received vehicle for up to 52 weeks (Fig. 3; Table 2). From week 4 to week 36, the proportion of participants maintaining ISGA score ≤ 1 was significantly greater (P < 0.05) in participants treated with crisaborole versus vehicle (Table 2).
Examples of AD lesions throughout the CrisADe CONTROL trial. a AD lesions on day 1/run-in baseline, b AD lesions at the end of the run-in period in participant classified as a responder, c flares during maintenance period, d resolved flares after treatment with crisaborole BID, e AD lesions at the end of the 52-week maintenance period. AD Atopic dermatitis, BID twice daily. Photographs were used with permission granted via informed consent/assent provided by each participant and/or their parent(s)/legal guardian(s)
When stratified by the duration of BID treatment in the open-label period (≤ 4 weeks and > 4 weeks), the proportion of crisaborole- versus vehicle-treated participants maintaining ISGA response was numerically greater in the ≤ 4 weeks subgroup throughout the 52-week maintenance period with the exception of week 20, when the adjusted difference was numerically greater in the > 4 weeks subgroup and week 24 when the adjusted difference was the same for the two subgroups (Table 3). Statistically, the proportion of participants maintaining ISGA response was significantly greater in participants treated with crisaborole versus vehicle from weeks 4 to 8 and from weeks 28 to 44 for the ≤ 4 weeks subgroup, while for the > 4 week subgroup, the proportion of crisaborole versus vehicle-treated participants maintaining ISGA response was significantly greater from weeks 4 to 24 (Table 3).
Severity of Flare
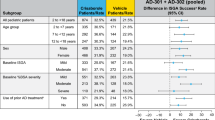
Of the 254 participants assessed during the maintenance period, 96 in the vehicle-treated group and 81 in the crisaborole-treated group had at least 1 flare. In the overall population there was a numerically lower percentage of participants with flares having ISGA scores of ≥ 2 among the crisaborole-treated group compared to the vehicle-treated group (Table 4).
When stratified by age the percentage of participants with an ISGA score of two flares for the ≥ 12 years subgroup was numerically less in crisaborole-treated versus vehicle-treated participants; however, for the 3 months to < 12 years subgroup, the percentage was numerically less in the vehicle-treated participants. The percentage of participants with an ISGA score of three flares for both age subgroups was numerically less in crisaborole-treated participants versus vehicle-treated participants. Across both treatment groups the incidence rate of flares with an ISGA score of 2 or 3 was lower in the ≥ 12 years subgroup compared to the < 12 years subgroup (Table 4).
When stratified by the duration of BID treatment during the open-label treatment period, the percentage of participants having a flare (ISGA = 2) for the ≤ 4 weeks subgroup was numerically lower in the vehicle-treated subgroup versus the crisaborole-treated subgroup; however, for the > 4 weeks subgroup the percentage was numerically lower in the crisaborole-treated subgroup. The percentage of participants with a flare (ISGA = 3) for both the ≤ 4 weeks and > 4 weeks subgroups was numerically lower in the crisaborole-treated group. Overall, there was a trend towards a lower incidence rate of flares having an ISGA score of 2 among the > 4 weeks subgroup, while the ≤ 4 weeks subgroup had a trend towards a lower incidence rate of flares having an ISGA score of 3 (Table 4).
Duration of Flare Periods
The median time until flare resolution was comparable between the crisaborole-treated and vehicle-treated participants in the overall population (54.0 vs. 54.1 days, respectively) and was similar between subgroups (Fig. 4). When stratified by age, the median time until flare resolution for the 3 months to < 12 years subgroup was numerically less in crisaborole-treated versus vehicle-treated participants; however, the median time in the ≥ 12 years subgroup for crisaborole-treated participants was numerically greater than that for vehicle-treated participants (Fig. 4). When stratified by the duration of BID treatment during the open-label treatment period, the median time until flare resolution for the ≤ 4 weeks subgroup was numerically less in crisaborole-treated versus vehicle-treated participants. In contrast, the median time in the > 4 weeks subgroup for crisaborole-treated participants was numerically greater than for vehicle-treated participants (Fig. 4).
Discussion
This exploratory analysis of the CrisADe CONTROL trial showed that adult and pediatric participants with mild to moderate AD at baseline who had achieved responder criteria (treatment success) with crisaborole BID during the run-in period maintained response per ISGA with crisaborole QD for ≤ 52 weeks during the double-blind maintenance period through week 52. Responses to crisaborole BID during the run-in period and to crisaborole QD during the maintenance period were similar between most subgroups.
From week 2 to week 6 of the open-label run-in period, ISGA response was achieved by a numerically greater proportion of participants in the age subgroup 3 months to < 12 years compared with the age subgroup ≥ 12 years and the overall population. This numerically greater response rate from week 2 to week 6 in younger patients may be explained by the difference in skin structure with age. Pediatric patients have a thinner stratum corneum than adults with increased permeability to topical agents [16,17,18] and a larger body surface area to weight ratio, both of which result in an increase in the proportion of drug absorbed via the skin in the former patient group as compared to adults [18]. In addition, chronic lichenified AD lesions are typically less frequent in children as compared to adults, which may also reduce absorption [19, 20].
Relief from AD-related signs and symptoms, maintenance of response following initial improvement, and consistency among diverse populations are important factors to consider when determining the optimal treatment option for patients with AD. Although there are several treatment options which provide patients with AD relief from the signs and symptoms associated with this skin condition, AD is characterized by a chronic recurrent course, and initial success is often difficult to maintain long term [9]. The results of the CrisADe CONTROL trial showed that crisaborole QD was effective for long-term maintenance treatment and flare reduction in adult and pediatric patients with mild to moderate AD over a 52-week period [15]. The exploratory analysis of the CrisADe CONTROL trial presented here demonstrated that the favorable initial treatment-related success (following an initial run-in period of up to 8 weeks with crisaborole BID) was sustainable with crisaborole QD during the 52-week maintenance period. During the double-blind maintenance period, the proportion of crisaborole- versus vehicle-treated participants maintaining ISGA response was generally numerically greater in participants who had an ISGA response in ≤ 4 weeks with BID treatment in the open-label period compared with those who took longer to have an ISGA response (> 4 weeks). A potential explanation would be that the subgroup with ≤ 4 weeks BID treatment had less severe AD at baseline.
The maintenance of ISGA response with crisaborole may be explained by the findings of a recent phase 2a, single-center, intrapatient, vehicle-controlled, proteomic analysis of 40 adults with mild to moderate AD and 20 healthy participants [21]. In that study, crisaborole induced normalization of the AD proteome towards a non-lesional non-AD normal skin molecular phenotype, supporting the role of topical PDE4 inhibition in the treatment of mild to moderate AD. Crisaborole may be useful as a broad-spectrum agent in AD management given its potential to target several general and immune pathways (e.g., Th2, Th17/Th22, and Th1 activation associated with AD pathogenesis in various stages and in different phenotypes of AD) [21,22,23].
The duration of flare periods during the maintenance period was found to be comparable for vehicle- and crisaborole-treated groups, with a numerically lower proportion of crisaborole-treated participants experiencing a flare with an ISGA score of ≥ 2 compared with vehicle-treated participants. Findings were comparable across most subgroups, including age subgroups. Previous studies have shown crisaborole to be effective for treating AD in patients aged ≥ 3 months with mild to moderate AD [12,13,14]. Likewise, the present exploratory analysis of the CrisADe CONTROL trial showed that the crisaborole-treated participants in age subgroups had consistent but comparable efficacy, emphasizing crisaborole’s consistency among different populations.
This study has a number of limitations. Firstly, the run-in period of the study was conducted in an open-label manner, which has the potential to introduce bias. Additionally, this study is limited by the lack of reporting on the resolution of flares between scheduled study visits, which might account for why the median time to flare resolution was similar in patients treated with crisaborole compared with those treated with vehicle. During flare treatment periods, participants were assessed every 4 weeks to determine if the flare had resolved (indicated by an ISGA score ≤ 1). However, in contrast to the process for detecting flares, instances of flare resolution occurring between scheduled study visits were not documented by patients or caregivers through electronic diaries or direct reporting. This lack of reporting between scheduled study visits during the flare period may have led to an overestimation of the duration of the reported flare periods. The small sample size of some of the subgroups was a further limitation. Further evaluations of crisaborole across larger subgroups representing diverse populations would provide valuable support for its use in all patients with AD. Finally, the study involved only post hoc and exploratory analyses.
Conclusions
This exploratory analysis showed that after initial treatment success with crisaborole BID, crisaborole QD provides a durable and consistent maintenance treatment by maintaining ISGA response in adult and pediatric participants with mild to moderate AD. The results were comparable among the age subgroups analyzed. Head-to-head efficacy and safety studies comparing crisaborole to first-line treatment options for AD may still be needed; however, the data from this expanded analysis on prior research further substantiate the role of crisaborole as maintenance treatment in AD.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2.
Bieber T. Atopic dermatitis Ann Dermatol. 2010;22:125–37.
Ständer S. Atopic dermatitis. N Engl J Med. 2021;384:1136–43.
Sidbury R, Alikhan A, Bercovitch L, et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89:e1–20.
Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema—part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022;36:1904–26.
Salava A, Perälä M, Pelkonen A, Mäkelä M, Remitz A. Safety of tacrolimus 0.03% and 0.1% ointments in young children with atopic dermatitis: a 36-month follow-up study. Clin Exp Dermatol. 2022;47:889–902.
Abędź N, Pawliczak R. Efficacy and safety of topical calcineurin inhibitors for the treatment of atopic dermatitis: meta-analysis of randomized clinical trials. Postepy Dermatol Alergol. 2019;36:752–9.
Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract. 2013;1(2):142–51.
Girolomoni G, Busà VM. Flare management in atopic dermatitis: from definition to treatment. Ther Adv Chronic Dis. 2022;13:20406223211066730.
Czarnowicki T, Gonzalez J, Bonifacio KM, et al. Diverse activation and differentiation of multiple B-cell subsets in patients with atopic dermatitis but not in patients with psoriasis. J Allergy Clin Immunol. 2016;137(118–29):e5.
Pfizer Laboratories Division, Pfizer Inc. Eucrisa® (crisaborole) ointment, for topical use. Highlights of prescribing information. 2023. https://labeling.pfizer.com/showlabeling.aspx?id=5331. Accessed 11 Mar 2024.
Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
Eichenfield LF, Call RS, Forsha DW, et al. Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J Am Acad Dermatol. 2017;77(641–9):e5.
Schlessinger J, Shepard JS, Gower R, et al. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to < 24 months with mild to moderate atopic dermatitis: a phase IV open-label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275–84.
Eichenfield LF, Gower RG, Xu J, et al. Once-daily crisaborole ointment, 2%, as a long-term maintenance treatment in patients aged ≥ 3 months with mild to moderate atopic dermatitis: a 52-week clinical study. Am J Clin Dermatol. 2023;15:1–13.
Oranges T, Dini V, Romanelli M. Skin physiology of the neonate and infant: clinical implications. Adv Wound Care (New Rochelle). 2015;4:587–95.
Kong F, Galzote C, Duan Y. Change in skin properties over the first 10 years of life: a cross-sectional study. Arch Dermatol Res. 2017;309(8):653–8.
O’Hara K. Pharmacokinetic changes with growth and development between birth and adulthood. J Pharm Pract Res. 2017;47:313–8.
Kolb L, Ferrer-Bruker SJ. Atopic dermatitis. StatPearls. Treasure Island: StatPearls Publishing LLC.; 2020.
Ramírez-Marín HA, Silverberg JI. Differences between pediatric and adult atopic dermatitis. Pediatr Dermatol. 2022;39:345–53.
Kim M, Del Duca E, Cheng J, et al. Crisaborole reverses dysregulation of the mild to moderate atopic dermatitis proteome toward nonlesional and normal skin. J Am Acad Dermatol. 2023;89:283–92.
Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139:S65–S76.
Guttman-Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:155–72.
Acknowledgements
We thank the participants of the study.
Medical Writing/Editorial Assistance
Editorial and medical writing support under the guidance of the authors was provided by Chantell Hayward, PharmD; Lisa Klumpp Callan, PhD; and Karis Moxley, PhD, at ApotheCom, San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022;175(9):1298–1304. doi: https://doi.org/10.7326/M22-1460).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study was sponsored by Pfizer Inc. Publication costs were funded by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
Conceptualization: Lawrence F. Eichenfield, Linda F. Stein Gold, Charles Lynde, Lyn Guenther, Shoshana Greenberger, Chia-Yu Chu, Zara Ghodsi, Bonnie Vlahos, Paul Sanders, Amy Cha, and Juliana M. Canosa contributed to the conceptualization of the study. Methodology: Lawrence F. Eichenfield, Linda F. Stein Gold, Charles Lynde, Lyn Guenther, Shoshana Greenberger, Chia-Yu Chu, Zara Ghodsi, Bonnie Vlahos, Paul Sanders, Amy Cha, and Juliana M. Canosa contributed to the development of the methodology for this post hoc analysis. Writing: Lawrence F. Eichenfield, Linda F. Stein Gold, Charles Lynde, Lyn Guenther, Shoshana Greenberger, Chia-Yu Chu, Zara Ghodsi, Bonnie Vlahos, Paul Sanders, Amy Cha, and Juliana M. Canosa contributed to draft development and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of Interest
Lawrence F. Eichenfield has served as a scientific advisor, consultant, and/or clinical trial investigator for Pfizer Inc., AbbVie, Amgen, Aslan Pharmaceuticals, Castle Biosciences, Dermavant, Eli Lilly and Company, Forté, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Regeneron Pharmaceuticals, and Sanofi-Genzyme. Linda F. Stein-Gold has received grants from Pfizer Inc., Incyte, and LEO Pharma and has received payment for lectures from Pfizer Inc. and LEO Pharma. Charles Lynde has been a speaker, consultant, and/or principal investigator for Pfizer Inc., AbbVie, Amgen, Aralez, Arcutis, Bausch Health, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cipher, Dermavant, Eli Lilly and Company, Fresnius Kabi, Galderma, GSK, InCyte, Innovaderm, Intega Skin, Janssen, Kyowa Kirin, La Roche-Posay, LEO Pharma, L’Oreal, Medexus, MedX, Merck, Novartis, P&G, Pediapharm, Regeneron, Roche, Sanofi-Genzyme, Sandoz, Sentrex, SunPharma, TEVA, Tribute, UCB, Valeant, Viatris, and Volo Health, and has served as a principal investigator for Acelyrin, Akros, Altius, Avillion, Celltrion, Concert, Devonian, Evelo, and MoonLake. Lyn Guenther has received consulting fees/honoraria from Pfizer Inc., Aralez, Bausch Health, BMS, Cipher, Eli Lilly and Company, GSK, Incyte, Janssen, Johnson and Johnson, La Roche-Posay, LEO Pharma, Merck, Miravo, Novartis, and UCB; has been a member of a Speaker’s Bureau/advisory board member for Pfizer Inc., Abbvie, Actilion, Amgen, Altana, Aralez, Aslan, Bausch Health, Eli Lilly and Company, Galderma, Janssen, Johnson and Johnson, La Roche-Posay, LEO Pharma, Novartis, and Sanofi Aventis, UCB; and has received research grants from Pfizer Inc., Abbvie, Actilion, Amgen, Bausch Health, BMS, Boehringer Ingelheim, Celgene, Cipher, Eli Lilly and Company, Galderma, Innovaderm, Janssen, La Roche-Posay, LEO Pharma, Merck, Novartis, and UCB. Shoshana Greenberger served as an investigator for Pfizer Inc., AbbVie, Amgen, Eli Lilly and Company, and, Novartis; has served on a Speaker’s Bureau/advisory board for Pfizer Inc., AbbVie, Sanofi, Pfizer, Padagis, and Dexcel Pharma; has provided investigator services for Pfizer Inc., AbbVie, Amgen, and Eli Lilly and Company; and has received research grants from Pfizer. Chia-Yu Chu has served as an investigator for Pfizer Inc., AbbVie, Amgen, Dermira, Eli Lilly and Company, Janssen, Novartis, Oneness Biotech, Regeneron, Roche, and Sanofi; has served as a consultant for Pfizer Inc., AbbVie, Eli Lilly and Company, Janssen, Novartis, Roche, and Sanofi; has served as a speaker for Pfizer Inc., AbbVie, Eli Lilly and Company, Janssen, Mylan, Novartis, Roche, Sanofi, and Viatris; and has served on the advisory boards of Pfizer Inc., AbbVie, Amgen, Eli Lilly and Company, Janssen, Mylan, Roche, Sanofi, and Viatris. Bonnie Vlahos, Paul Sanders, Amy Cha, Juliana M. Canosa, and Zara Ghodsi are employees and shareholders of Pfizer Inc.
Ethical Approval
This planned exploratory analysis of data from the CrisADe CONTROL study was exempt from institutional review board approval. All participants or parents/guardians provided written informed consent for participation in the CrisADe CONTROL study. The CrisADe CONTROL study was approved by the Quorum Review Institutional Review Board and was conducted in accordance with the ethical principles originating in the Declaration of Helsinki.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Eichenfield, L.F., Stein Gold, L.F., Lynde, C. et al. Maintenance of Investigator’s Static Global Assessment Response with Once-Daily Crisaborole in Participants with Mild to Moderate Atopic Dermatitis. Dermatol Ther (Heidelb) 14, 875–892 (2024). https://doi.org/10.1007/s13555-024-01129-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01129-9