Abstract
Introduction
Psoriasis is a chronic, immune-mediated inflammatory skin disease. Despite the availability of several therapies, many patients affected by this disease remain untreated, do not have adequate response, or suffer from treatment-related toxic effects. It has been shown that the interleukin (IL)-17 pathway plays a key role in the immunopathogenesis of psoriasis. Brodalumab, the first human monoclonal IgG2 antibody that selectively binds to subunit A of the human IL-17 receptor, blocking interactions with a number of cytokines of the IL-17 family, has confirmed fast onset of action, high complete clearance rates, and sustained efficacy. Nevertheless, there is only a limited amount of published real-world evidence (RWE) data.
Methods
This was an open-label, multicenter, real-world, prospective, non-interventional, non-controlled (single-arm) observational study (LIBERO-CZ) assessing the management of moderate to severe psoriasis with brodalumab in daily practice for up to 52 weeks of treatment.
Results
Fifty-four patients (70.4% male, mean age 46.9 ± 13.4 years, weight 95.6 ± 22.7 kg, disease duration 18.6 ± 12.7 years) were enrolled and included in the final analysis. Forty-nine of the patients completed the study and five discontinued prematurely; 51.8% of all the enrolled patients were biologic-naïve. At baseline, 28% patients were classified as severe (psoriasis area severity index (PASI) ≥ 20). Overall, the mean PASI decreased by 15.6 from 16.1 (± 5.0) at baseline to 0.5 (± 1.2) at the last visit. The primary endpoint of an absolute PASI ≤ 3 at week 12 (as observed analysis) was achieved by 95.9% of patients. The static Physician’s Global Assessment (sPGA) success (defined as clear = 0 and almost clear = 1) at week 52 was achieved by 92.1% of patients. PASI 75, PASI 90, and PASI 100 were achieved by 98.0%, 87.8%, and 75.5% of patients, respectively, after approximately 52 weeks of treatment. The study also recorded very positive results concerning patient-reported outcomes.
Conclusions
LIBERO-CZ confirms the fast onset and high clearance rates of brodalumab in real life in both biologic-naïve and biologic-experienced patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Psoriasis is a chronic, immune-mediated inflammatory skin disease. Although not a life-threatening disease, the related physical and psychological burden means psoriasis has a major impact on the quality of life of patients. |
There is only a limited amount of published real-world evidence (RWE) data for brodalumab. |
What was learned from this study? |
The therapy of psoriasis with brodalumab was effective and tolerable and resulted in significantly reduced psoriasis severity and symptoms in daily practice. |
The primary endpoint of an absolute Psoriasis Area Severity Index (PASI) ≤ 3 after 12 weeks of treatment was achieved by 95.9% of patients. The static Physician’s Global Assessment (sPGA) success (defined as clear = 0 and almost clear = 1) at week 52 was achieved by 92.1% of patients. |
Patients attested to a clear benefit from the therapy, including an improvement in their quality of life and a high level of satisfaction with the therapy. |
Introduction
Psoriasis is a chronic, immune-mediated inflammatory skin disease, characterized by proliferation of keratinocytes and accumulation of immune cells in the affected skin, with an estimated prevalence of 2% in the European adult population [1]. Although not a life-threatening disease, as a result of its physical and psychological burden, psoriasis has a major impact on the quality of life [2]. The genetic background of psoriasis has been well described with the involvement of psoriasis susceptibility locus 1 (PSOR1), but also with other genes which remain under investigation like ATG16L1 [3]. Despite the availability of several therapies, many patients remain untreated, do not have an adequate response, or have treatment-related toxic effects [4]. Therapy needs to be highly effective and safe in the short and long term, which is achievable through biologic therapy. Nevertheless, it is imperative to understand the major benefits and limitations of different biologic drugs. Certain types of therapy may be more suitable for a specific subset of patient population and not all patients may respond well to the initial choice of biologic drug [5].
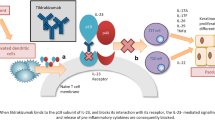
The interleukin (IL)-17 pathway plays a key role in the immunopathogenesis of psoriasis [6]. Genome-wide association studies have linked IL-17 pathway-related genes with psoriasis [7, 8], and IL-17 mRNA levels are higher in psoriatic lesions than in normal skin [9, 10]. Numbers of T helper (h) 17 cells are increased in psoriatic lesions and are stimulated by IL-23 to release IL-17 cytokines [11]. IL-17 cytokines like IL-17A, IL-17C, IL- 17F, and IL-17A/F can induce the expression of psoriasis-related proinflammatory molecules in keratinocytes, leading to the recruitment and accumulation of neutrophils, T cells, and dendritic cells [12,13,14].
Brodalumab is the first human monoclonal IgG2 antibody that selectively binds to the human IL-17A receptor and thereby blocks its interactions with a number of cytokines of the IL-17 family (IL-17A, C, E, F) [15,16,17]. Brodalumab has been available in Europe since 2017 under the brand name Kyntheum® for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
The approval of brodalumab was based on three randomized, double-blind, placebo-controlled phase 3 trials including a 12-week induction phase followed by withdrawal/re-treatment or a maintenance phase. AMAGINE-1 (n = 661) evaluated the efficacy, safety, withdrawal, and re-treatment effect of brodalumab compared with placebo. AMAGINE-2 (n = 1831) and AMAGINE-3 (n = 1881) were 12-week induction trials followed by re-randomization at week 12 and evaluated the efficacy and safety of induction and maintenance of brodalumab compared with ustekinumab and placebo. Results showed that brodalumab offered more patients complete skin clearance measured by improvement of the Psoriasis Area Severity Index by 100% (PASI 100) at 12 weeks compared to patients treated with ustekinumab [AMAGINE-2: 44% (n = 272) versus 22% (n = 65), p < 0.001; AMAGINE-3: 37% (n = 229) versus 19% (n = 58), p < 0.001] [18,19,20,21].
Real-world evidence (RWE) provides valuable information for a better understanding of the attributes in different patients. The purpose of this non-interventional observational study (NIS) was to assess the short- and long-term management outcome of patients with moderate to severe psoriasis treated with brodalumab in daily practice, by assessing the proportion of patients achieving an absolute PASI ≤ 3 at week 12 and, in patients who continued after 12 weeks of treatment, the proportion of patients achieving the static Physician’s Global Assessment (sPGA) success (defined as clear/almost clear) at week 52. The secondary objective was to describe different patient profiles managed with brodalumab in the diversity of a real-world setting regarding demographics, disease severity, previous treatment regimen and satisfaction with it. Patient-reported outcomes were also collected, to create substantial insights regarding the patient’s benefit as well as satisfaction with the therapy.
Methods
Study Setting and Patient Population
This was an open-label, multicenter, real-world, 12- and 52-week, prospective, non-controlled (single-arm) observational study. Centers which took part in this study were dermatology departments in Czech Republic university hospitals. All patients signed informed consent. The study was non-interventional and therefore the assignment of a patient to brodalumab was according to local regulations. The prescription of brodalumab was clearly separated from the decision to include the patient in the study. There was also no additional diagnostic or monitoring procedure applied to the patients, and only epidemiological methods were used for the analysis of collected data. The study was conducted in accordance with the Helsinki Declaration of 1964 and all subsequent amendments, and all patients provided written informed consent. Patient-level data used for this analysis were de-identified. The study was approved by ethical committees of the appointed Institutions (Královské Vinohrady University Hospital, General University Hospital in Prague, St. Anne’s University Hospital Brno, University Hospital in Pilsen, and Motol University Hospital). The study was officially reported to the State Institute for Drug Control, Czech Republic, under the following identification number: 1903110001.
Prospective data collection was performed by the participating dermatologists during routine patient care at the time of first injection of brodalumab (baseline) and approximately (proposed intervals based on knowledge of standard clinical practice in cooperation with local experts, fully adaptable by any participating physician) at the following time intervals: weeks 2, 4, 12, 24, 36, 48, and 52 (close-out visit). Data have been collected by physicians, but also directly by patients.
For physicians, data were collected from the standard medical records of the participating centers. At baseline, physicians completed a questionnaire on the patient’s demographics, physical examination, psoriasis and medical history, prior and current psoriasis treatment, concomitant medication, and—for women of childbearing age—pregnancy status/pregnancy prevention control method, respectively. If available, relevant laboratory testing results were collected. At all visits the clinical psoriasis status was assessed by describing psoriasis type and characteristics, affected areas, body surface area (BSA), PASI, and the sPGA (6-point scale, ranging from 0 = clear skin to 5 = very severe disease; a score of 3 indicates moderate disease). Date of administration of brodalumab and any change in psoriasis or concomitant medication, diseases, and—for a woman of childbearing age—pregnancy status were recorded.
Concerning data collected by patients, the static Patient’s Global Assessment (PaGA) mirroring the sPGA 6-point scale (0 = clear skin to 5 = very severe disease; a score of 3 indicates moderate disease) was assessed at each visit. At baseline, at the end of the induction phase (after 12 weeks of treatment) and during the last visit, patients were asked to answer a short series of questionnaires including the Dermatological Life Quality Index (DLQI) [22], Quality of Life Enjoyment and Satisfaction (Q-LES-Q-SF) [23], general and emotional well-being, Patient Benefit Index (PBI) [24], and their satisfaction with previous and current therapy, Treatment Satisfaction Questionnaire for Medication (TSQM-9) [25].
This study included adult patients, who were candidates for systematic therapy with brodalumab, as per the SmPC and local guidelines [26, 27].
Analysis
Considering the non-interventional character of the study, a statistical analysis was performed in a descriptive and explorative way. No hypothesis testing was conducted, and data were summarized with respect to demographic and baseline characteristics. The continuous variables were described by the number of valid (evaluable) cases, mean, standard deviation (SD), minimum and maximum, median, and lower and upper quartiles. Categorical variables were described by the number of cases, frequency, and percentage. The 95% confidence intervals were computed where suitable. Graphical representations of efficiency variables were prepared, if relevant. Changes from the baseline were analyzed using paired tests.
Results
Patient Demographics and Previous Treatments
Between July 2019 and March 2022, a total of 54 patients, 38 (70.4%) men and 16 (29.6%) women, from six sites in the Czech Republic, with mean ± SD disease duration of 18.6 ± 12.7 years, were enrolled and included in the final analysis and evaluation. The mean age of the patients at the time of brodalumab treatment initiation was 46.9 ± 13.4 years. The mean weight was 95.6 ± 22.7 kg, and mean BMI was 32.6 ± 18.4; 79.7% of patients were overweight (BMI ≥ 25), and 51.8% of patients were obese (BMI ≥ 30).
Forty-nine of all the enrolled patients, 35 (71.4%) men, 14 (28.6%) women, mean age 46.2 ± 13.6 years, weight 94.0 ± 21.7 kg, disease duration 19.3 ± 12.9 years, completed all the visits and thus approximately 52 weeks of treatment. Five patients prematurely discontinued because of insufficient effectiveness (3.7%; n = 2), intolerance (1.9%; n = 1), or the patient did not attend the physician visit for any additional treatment (1.9%; n = 1). For one of the patients, the reason for premature discontinuation was missing. The mean treatment duration was 370.5 ± 89.5 days. Considering the socioeconomic status of the enrolled patients, most of them were married (33; 61.1%), employed (44; 81.48%), and had high-school and higher education (44; 81.5%). Concerning the baseline comorbidities, 35 patients (64.8%) were enrolled without concomitant diseases. Seven (13.0%) patients had other manifestation of psoriasis, mainly nail (4; 7.3%) or inverse psoriasis (2; 3.6%). Table 1 presents the demographic and clinical data of the study patient population, including comorbidities and previous treatments.
Previous therapy consisted mainly of systemic treatment with methotrexate (74.1%), retinoids (55.6%), and cyclosporine (35.2%), respectively. Twenty-six (48.2%) of all the enrolled patients were biologic naïve. Thirteen of all the enrolled patients had been previously treated with one biologic (24.1%), four patients with two biologics (7.4%), and nine patients with three or more biologics (16.7%), mainly with adalimumab (31.5%), etanercept (16.7%), secukinumab (14.8%), or ustekinumab (11.1%). Details concerning last treatment with biologics/biosimilars are presented in Fig. 1.
Data Collected by Physicians
The reason for starting the therapy with brodalumab was mainly lack of efficacy of the previous therapy (59.4% of patients), severity of the disease (31.9%), therapy refractory course under previous therapies (5.8%), or insufficient compatibility/contraindications of previous treatment (2.9%).
At baseline, there were 28.6% patients with PASI ≥ 20 and 69.4% patients with PASI ≥ 10 and < 20. Overall, the mean PASI decreased from 16.1 (± 5.0) at baseline to 7.2 (± 5.1) at week 2 and further improved to 1.0 at week 12 (± 3.4). After 52 weeks of treatment, the mean PASI was 0.5 (± 1.2). When we look at the subgroups, for severe/very severe patients (baseline PASI ≥ 20), the mean PASI decreased by 21.5 from 22.3 (± 4.3) at baseline to 0.8 (± 1.7) at week 52. For patients with baseline PASI < 20, the absolute change was 13.3, and the absolute PASI decreased from 13.7 (2.6) to 0.4 (0.8). The number of patients who achieved a PASI score of ≤ 3 points increased continuously during the NIS, achieving 95.9% after 52 weeks of treatment; 95.9% of patients also met the primary endpoint of an absolute PASI ≤ 3 at week 12. The percentage of patients achieving PASI ≤ 2 and ≤ 1 was 91.8% and 83.7%, respectively, at week 12. Absolute PASI results are summarized in Fig. 2.
Percentage of patients achieving absolute Psoriasis Area Severity Index (PASI) values of ≤ 3, 2, and 1 in the course of the study; 95.9% of patients met the primary endpoint of an absolute PASI ≤ 3 at week 12. The percentage of patients achieving PASI ≤ 2 and ≤ 1 was 91.8% and 83.7%, respectively, at week 12
At week 12, PASI 75 was achieved in 49 patients (92.5%), PASI 90 in 45 patients (84.9%), and PASI 100 at 32 (60.4%) patients. Furthermore, 98.0% patients achieved PASI 75, 87.8% PASI 90, and 75.5% PASI 100 after 52 weeks of treatment. Results for the total group and relevant subgroups are presented in Table 2. The highest PASI 100 response rates after 52 weeks of therapy with brodalumab were observed in patients with moderate severity at enrollment. Efficacy of brodalumab was observed in both biologic-naïve patients and those previously treated by anti-TNFα, anti-IL-17A, or IL-23/12 or IL-23. Figure 3 summarizes the mean PASI progress in all patients and individual subgroups.
Mean absolute PASI. During the therapy with brodalumab, the mean PASI decreased from 16.1 (± 5.0) at baseline to 1.0 (± 3.4) at week 12. After 52 weeks of treatment, the mean PASI was 0.5 (± 1.2). Concerning the individual subgroups, efficacy of brodalumab, evaluated on the based of absolute PASI change, was observed in both biologic-naïve patients and those previously treated by anti-TNFα, anti-IL-17A, or IL-23/12 or IL-23. BL baseline, Bx biologic, IL interleukin, PASI Psoriasis Area Severity Index, TNFα tumor necrosis factor alpha
According to the sPGA, the severity of psoriasis improved continuously during treatment with brodalumab. At the start of the study, the physicians rated the severity with an average of 3.6 (± 0.6) points, and 50.0% of patients were severe, 43.8% moderate, and 6.2% very severe. At the end of the study, the average sPGA score was 0.4 (± 0.6) points, a reduction of 3.2 points. sPGA 0/1 at week 52 was achieved by 92.1%, with 93.3% of patients achieving this at week 12 (Fig. 4).
The mean body surface area (BSA) decreased during treatment with brodalumab by 20.1%-points from 20.8 (± 10.6) % before the start of therapy to 0.7 (± 1.9) % at the last visit (Fig. 5).
Data Collected by Patients
On average, the participants rated the severity of their disease by the PaGA with 3.7 (± 0.7; median 4.0) points at the beginning of the NIS. After 12 weeks the value was 0.7 (± 0.8; median 0.5). Finally, after 52 weeks of treatment, the average value was 0.5 (± 0.7; median 0.0) points, a reduction of 3.2 points.
Treatment, according to the Patient Benefit Index (PBI), helped most to lead a normal working life with a mean of 3.5 points after 12 weeks of treatment and 3.9 at the final visit. The overall benefit according to the PBI was classified as fairly or very (more than 3 points) beneficial for 55.1% of the patients after about 12 weeks and for 71.4% after about 52 weeks. The mean overall benefit as per PBI was 2.7 ± 1.4 at week 12 and 3.1 ± 1.3 at the last visit (week 52).
The mean value of the Dermatology Life Quality Index (DLQI) at the beginning of the NIS was 14.5 (± 6.5; median 14.0). During therapy with brodalumab the mean value decreased by 12.4 to 2.1 (± 3.15) at week 12. At the last visit the mean value was 1.1 (± 1.9), reduced by 13.4 from baseline. After approximately 12 weeks of treatment, 65.2% no longer had any impairment in the quality of life (DLQI 0 or 1) nor at the end of the study. This proportion was 75.6% (Fig. 6).
DLQI category during progress of the study. During therapy with brodalumab the mean value decreased by 12.4 to 2.1 (± 3.15) at week 12. At the last visit the mean value was 1.1 (± 1.9), reduced by 13.4 from baseline. DLQI 0 or 1 ≈ no impairment in the quality of life. DLQI Dermatological Life Quality Index
After approximately 52 weeks of treatment, the Treatment Satisfaction Questionnaire for Medication (TSQM-9) average score for efficacy was 87.9 (± 18.8), while ease of use was rated at an average of 83.0 (± 11.4). The reported mean for overall satisfaction with brodalumab was 88.8 (± 10.0).
As regards the Q-LES-Q-SF, the raw total score after approximately 52 weeks was on average 58.6 (± 5.4), and 79.6% when transformed into a percentage. At the end of the treatment most patients mentioned their satisfaction with the medication (average 4.3 ± 0.6) and the rating of satisfaction and overall contentment with life was also high (average 4.3 ± 0.5). Figure 7 displays the results in graphical form for TSQM-9 and Q-LES-Q-SF.
Treatment Satisfaction Questionnaire for Medication (TSQM-9) and the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q-SF) progress during the study. After approximately 52 weeks of treatment, the reported mean for overall satisfaction with brodalumab (TSQM-9) was 88.8 (± 10.0). For Q-LES-Q-SF, total score was on average 58.6 (± 5.4), and 79.6% when transformed into a percentage
Safety Results
During the study, there were in total 25 reports of adverse events (AE) for which a connection with the brodalumab therapy could not be ruled out. Of these 25 reported cases, the most common was COVID-19 (six cases, 24.0%), diarrhea (three cases, 12.0%), followed by arthralgia (8.0%) and common cold (8.0%). All other AEs were documented with a frequency of less than 5%. Five events (20.0%) were classified as serious. The reported serious adverse events included ulcerative colitis, erysipelas, bronchitis, epiretinal membrane with cystoid macular edema, and bilateral viral pneumonia with COVID-19.
Discussion
In this multicenter, real-world, prospective, non-controlled (single-arm) observational study we demonstrated the fast onset and high clearance rates of brodalumab in real life in both biologic-naïve and biologic-experienced patients.
Analysis of demographic data showed a higher prevalence of psoriasis in men, what is in line with data from several registries, including those from Denmark (64.5%) [28], the UK (60.3%) [29], Spain (58.9%) [30], Hungary (61.3) [31], Germany (60.0%) [32], and USA (52.0%) [33]. Similar numbers have been also described in the Czech Republic registry for biological treatment (BIOREP) [34], representing the local environment. A higher percentage of patients with overweight/obesity was shown in our study: the percentage of patients with overweight or obesity (BMI ≥ 25) was 79.6% and the percentage of patients with elevated WHR (male, ≥ 0.9; female, ≥ 0.8) was 80.0%.
There was a high mean onset age at the initiation of biologic therapy (46.9 ± 13.4) within this study, while duration of disease was 18.6 years (time from the first diagnosis of psoriasis). Generally, in patients with moderate to severe psoriasis, there is a substantial delay before the initiation of systemic treatment [35]. Data from registries show long periods of untreated psoriasis, which range from 15 years in Spain up to 22 years in the UK [28,29,30, 32]. In the Czech Republic the mean duration of psoriasis prior to biological treatment is 21.6 years [36].
The percentage of biologic-naïve patients in our study was quite high (48.2%). This can be explained by the fact that physicians would prefer to initiate biologic therapy with a drug showing a high response rate [37].
Despite the fact that patients in this study started the biological therapy of psoriasis with brodalumab more than 18 years after diagnosis of psoriasis and had both high disease severity and low quality of life, the real-world effectiveness of brodalumab was high with 95.9% of patients achieving the primary endpoint of an absolute PASI ≤ 3 after 12 weeks of treatment.
Our results regarding PASI scores showed rapid improvement immediately after the commencement of brodalumab therapy. The mean PASI decreased from 16.1 (± 5.0) at baseline to 7.2 (± 5.1) at week 2 and further improved to 1.0 at week 12 (± 3.4). The same trend has been seen in the full study sample, but also for individual subgroups. After 12 weeks of treatment, PASI 75 was achieved in 92.5%, PASI 90 in 84.9%, and PASI 100 in 60.4% of patients. Our results showed a higher percentage of PASI 90 and PASI 100 at week 12 in comparison with the study by Gargiulo et al., representing real-life data from the Italian cohort, where PASI 90 was achieved by 63.5% and PASI 100 by 49.2% in week 12 [38]. Our numbers subsequently improved with up to 98.0% patients achieving PASI 75, 87.8% PASI 90, and 75.5% PASI 100 after 52 weeks of treatment.
Similarly, other results from this study also confirm the fast onset and long-term duration of brodalumab’s effect. According to the sPGA, more than 90% of the participants achieved a clear or almost clear score at as early as 12 weeks of brodalumab treatment, keeping this percentage till the end of the study. After 52 weeks of treatment, the mean PASI was 0.5 and the mean BSA decreased during treatment by more than 20%-points to 0.7. Similar results were obtained in a study on real clinical practice in Andalucia, Spain, where the mean PASI in week 52 was 1.3 and mean BSA 2.3 [39]. Papadavid et al. analyzed 106 patients receiving brodalumab in Greece and they also showed a significant reduction of PASI score (14.0–1.5, p < 0.001) and BSA scores (21.6–2.5, p < 0.001) across all visits [40]. It is also necessary to mention that in comparison with the data from clinical trials, the efficacy results gathered within our study were much better, which is of course reflecting the RWE setup and local specifics.
Psoriasis has a well-known negative impact on the quality of life of patients, even if only residual lesions remain in the skin. Patients achieving PASI 100 response have a higher chance of achieving DLQI 0/1 than those with PASI 90 response. Complete and long-term clearance is considered to be the ideal treatment target [41,42,43]. Our patients showed a significant decrease in the DLQI score during brodalumab therapy, from 14.5 to 2.1 after approximately 12 weeks of treatment. These results are comparable with the median score change on DLQI, which was achieved in the study focused on effectiveness of brodalumab in patients with moderate to severe plaque psoriasis in the Greek clinical setting, where DLQI decreased from 12.9 at baseline to 3.8 at week 12–16 [44]. In our study, the mean value at the last visit was 1.1, a reduction of 13.4 from baseline. Even though the study did not test any hypothesis, there was an indication of a relationship between the DLQI and mean absolute PASI, starting at week 12.
After approximately 52 weeks, the TSQM-9 average score for efficacy was 87.9 (± 18.8), while ease of use was rated at an average of 83.0 (± 11.4). The reported mean for overall satisfaction with brodalumab was 88.8 (± 10.0). In the publication by Imafuku et al., who analyzed Japanese patients on brodalumab, a significant improvement of TSQM-9 domain was observed in week 12 and 40 [45].
Concerning the Q-LES-Q-SF, the raw total score after approximately 52 weeks was on average 58.6 (± 5.4), and 79.6% when transformed into a percentage. At the end of the treatment, most of the patients mentioned their satisfaction with the medication (average 4.3 ± 0.6) and the rating of satisfaction and overall contentment with life was also high (average 4.3 ± 0.5). Q-LES-Q-SF is a 16-item self-administered questionnaire that captures life satisfaction over the past week. Since psoriasis is a disease with high impact on quality of life and psychosocial activities, we decided to use this questionnaire to monitor the effect of therapy on life enjoyment and satisfaction.
The safety profile of brodalumab in our patients was like that reported in clinical trials [18, 21]. No new safety signals were observed.
A limitation of this study is the absence of a control group, which is typical for real-world data. In addition, the number of participants was not very high.
Conclusion
The present non-interventional study analyzed representative data on the efficacy, safety, and tolerability of brodalumab 210 mg in a cohort of 54 patients. Insights were gained into when and how brodalumab are used under real-world conditions, specifically in the Czech Republic environment. The therapy of psoriasis with brodalumab proved to be effective and tolerable in everyday treatment and resulted in significantly reduced psoriasis severity and symptoms.
As early as 12 weeks there was a clear response to the therapy, which was also be maintained with long-term use at 52 weeks. The patients also attested to a clear benefit from the therapy, including an improvement in their quality of life and a high level of satisfaction with the therapy.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. https://doi.org/10.1136/bmj.m1590.
Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28(3):333–7. https://doi.org/10.1111/jdv.12106.
Douroudis K, Kingo K, Traks T, et al. Polymorphisms in the ATG16L1 gene are associated with psoriasis vulgaris. Acta Derm Venereol. 2012;92(1):85–7. https://doi.org/10.2340/00015555-1183.
Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014 May;70(5):871-81.e1-30. https://doi.org/10.1016/j.jaad.2013.12.018.
Kim HJ, Lebwohl MG. Biologics and psoriasis: the beat goes on. Dermatol Clin. 2019;37(1):29–36. https://doi.org/10.1016/j.det.2018.07.004.
Beringer A, Noack M, Miossec P. IL-17 in chronic inflammation: from discovery to targeting. Trends Mol Med. 2016;22(3):230–41. https://doi.org/10.1016/j.molmed.2016.01.001.
Elder JT. Genome-wide association scan yields new insights into the immunopathogenesis of psoriasis. Genes Immun. 2009;10(3):201–9. https://doi.org/10.1038/gene.2009.11.
Ellinghaus E, Ellinghaus D, Stuart PE, et al. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet. 2010;42(11):991–5. https://doi.org/10.1038/ng.689.
Li J, Li D, Tan Z. The expression of interleukin-17, interferon-gamma, and macrophage inflammatory protein-3 alpha mRNA in patients with psoriasis vulgaris. J Huazhong Univ Sci Technolog Med Sci. 2004;24(3):294–6. https://doi.org/10.1007/BF02832018.
Teunissen MB, Koomen CW, de Waal MR, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111(4):645–9. https://doi.org/10.1046/j.1523-1747.1998.00347.x.
Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128(5):1207–11. https://doi.org/10.1038/sj.jid.5701213.
Johnston A, Fritz Y, Dawes SM, et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol. 2013;190(5):2252–62. https://doi.org/10.4049/jimmunol.1201505.
Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159(5):1092–102. https://doi.org/10.1111/j.1365-2133.2008.08769.x.
Ramirez-Carrozzi V, Sambandam A, Luis E, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12(12):1159–66. https://doi.org/10.1038/ni.2156.
Campa M, Mansouri B, Warren R, Menter A. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis. Dermatol Ther (Heidelb). 2016;6(1):1–12. https://doi.org/10.1007/s13555-015-0092-3. (Erratum in: Dermatol Ther (Heidelb). 2016 Jun;6(2):305).
Coimbra S, Figueiredo A, Santos-Silva A. Brodalumab: an evidence-based review of its potential in the treatment of moderate-to-severe psoriasis. Core Evid. 2014;21(9):89–97. https://doi.org/10.2147/CE.S33940.
Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192(8):3828–36. https://doi.org/10.4049/jimmunol.1301737.
Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–86. https://doi.org/10.1111/bjd.14493.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–28. https://doi.org/10.1056/NEJMoa1503824.
Gordon KB, Kimball AB, Chau D, et al. Impact of brodalumab treatment on psoriasis symptoms and health-related quality of life: use of a novel patient-reported outcome measure, the Psoriasis Symptom Inventory. Br J Dermatol. 2014;170(3):705–15. https://doi.org/10.1111/bjd.12636.
Attia A, Abushouk AI, Ahmed H, et al. Safety and efficacy of brodalumab for moderate-to-severe plaque psoriasis: a systematic review and meta-analysis. Clin Drug Investig. 2017;37(5):439–51. https://doi.org/10.1007/s40261-017-0500-9.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6. https://doi.org/10.1111/j.1365-2230.1994.tb01167.x.
Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–6.
Feuerhahn J, Blome C, Radtke M, Augustin M. Validation of the patient benefit index for the assessment of patient-relevant benefit in the treatment of psoriasis. Arch Dermatol Res. 2012;304(6):433–41. https://doi.org/10.1007/s00403-012-1256-y.
Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;27(7):36. https://doi.org/10.1186/1477-7525-7-36.
Cetkovska P, Kojanova M, Arenberger P, Fabianova J. Současný stav moderní léčby psoriázy – aktualizovaná doporučení ČDS JEP k cílené léčbě závažné chronické psoriázy. Čes-slov Derm. 2019;94(4):135–64.
European Commission, Union Register of medicinal products for human use, Kyntheum® (brodalumab). http://ec.europa.eu/health/documents/community-register/html/h1155.htm. Accessed Sep 2023.
Gniadecki R, Bang B, Bryld LE, Iversen L, Lasthein S, Skov L. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol. 2015;172(1):244–52. https://doi.org/10.1111/bjd.13343.
Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632–40. https://doi.org/10.1038/jid.2015.208.
Belinchón I, Ramos JM, Carretero G, et al. Adverse events associated with discontinuation of the biologics/classic systemic treatments for moderate-to-severe plaque psoriasis: data from the Spanish Biologics Registry, Biobadaderm J Eur Acad Dermatol Venereol. 2017;31(10):1700–8. https://doi.org/10.1111/jdv.14314.
Pogácsás L, Borsi A, Takács P, et al. Long-term drug survival and predictor analysis of the whole psoriatic patient population on biological therapy in Hungary. J Dermatolog Treat. 2017;28(7):635–41. https://doi.org/10.1080/09546634.2017.1329504.
Reich K, Mrowietz U, Radtke MA, et al. Drug safety of systemic treatments for psoriasis: results from The German Psoriasis Registry PsoBest. Arch Dermatol Res. 2015;307(10):875–83. https://doi.org/10.1007/s00403-015-1593-8.
Strober B, Karki C, Mason M, et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: results from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2018;78(2):323–32. https://doi.org/10.1016/j.jaad.2017.10.012.
Kojanova M, Fialova J, Cetkovska P, et al. Characteristics and risk profile of psoriasis patients included in the Czech national registry BIOREP and a comparison with other registries. Int J Dermatol. 2017;56(4):428–34. https://doi.org/10.1111/ijd.13543.
Maza A, Richard MA, Aubin F, et al. Significant delay in the introduction of systemic treatment of moderate to severe psoriasis: a prospective multicentre observational study in outpatients from hospital dermatology departments in France. Br J Dermatol. 2012;167(3):643–8. https://doi.org/10.1111/j.1365-2133.2012.10991.x.
Kojanova M, Fialova J, Cetkovska P, . Demographic data, comorbidities, quality of life, and survival probability of biologic therapy associated with sex-specific differences in psoriasis in the Czech Republic. Dermatol Ther. 2021;34(2):e14849. https://doi.org/10.1111/dth.14849.
Adenubiova E, Arenberger P, Gkalpakioti P, et al. Psoriasis treatment with adalimumab in clinical practice: long-term experience in a center for biological therapy in the Czech Republic. J Dermatolog Treat. 2018;29(6):579–82. https://doi.org/10.1080/09546634.2018.1425358.
Gargiulo L, Ibba L, Malagoli P, et al. Brodalumab for the treatment of plaque psoriasis in a real-life setting: a 3 years multicenter retrospective study-IL PSO (Italian landscape psoriasis). Front Med (Lausanne). 2023;3(10):1196966. https://doi.org/10.3389/fmed.2023.1196966.
Galan-Gutierrez M, Font-Ugalde P, Padilla L, et al. Brodalumab: efficacy, safety, and survival in mid-term (52 weeks) on real clinical practice in Andalucia. Spain Int J Dermatol. 2023;62(5):700–6. https://doi.org/10.1111/ijd.16527.
Papadavid E, Zafeiriou E, Georgiou S, et al. Real-world clinical outcomes of treatment with brodalumab in patients with moderate-to-severe psoriasis: a retrospective, 24-month experience from four academic dermatology centers in Greece. J Dermatolog Treat. 2022;33(7):3053–9.
Puig L, Thom H, Mollon P, Tian H, Ramakrishna GS. Clear or almost clear skin improves the quality of life in patients with moderate-to-severe psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2017;31(2):213–20. https://doi.org/10.1111/jdv.14007.
Strober B, Papp KA, Lebwohl M, et al. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol. 2016;75(1):77–82.e7. https://doi.org/10.1016/j.jaad.2016.03.026.
Takeshita J, Callis Duffin K, Shin DB, et al. Patient-reported outcomes for psoriasis patients with clear versus almost clear skin in the clinical setting. J Am Acad Dermatol. 2014;71(4):633–41. https://doi.org/10.1016/j.jaad.2014.05.001.
Rigopoulos D, Angelakopoulos C, Apalla Z, et al. Real world experience of brodalumab treatment in patients with moderate-to-severe plaque psoriasis in the Greek population: Results from an interim analysis of the BrIDGE study. Dermatol Ther. 2022;35(12):e15886. https://doi.org/10.1111/dth.15886.
Imafuku S, Ohata C, Okubo Y, et al. Effectiveness of brodalumab in achieving treatment satisfaction for patients with plaque psoriasis: the ProLOGUE study. J Dermatol Sci. 2022;105(3):176–84. https://doi.org/10.1016/j.jdermsci.2022.02.007.
Acknowledgements
The authors wish to thank all of the dermatologists and collaborators who participated in the study LIBERO Czech Republic for their efforts and dedication to the project.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study and the Rapid Service Fee were funded by LEO Pharma.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study analysis. Spyridon Gkalpakiotis drafted the article. Martina Kojanová, Jorga Fialová, Petra Cetkovská, Vladimír Vašků, Yvetta Vantuchová, Alena Machovcová, Petra Gkalpakioti, Pavla Hrdá and Petr Arenberger reviewed the paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
Spyridon Gkalpakiotis, Martina Kojanová, Jorga Fialová, Vladimír Vašků, Petra Gkalpakioti, Petra Cetkovská, Alena Machovcová, Yvetta Vantuchová and Petr Arenberger have served as consultants, speakers, or investigators for Abbvie, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi and UCB. Pavla Hrda is an employee of LEO Pharma.
Ethical Approval
The study was conducted in accordance with the Helsinki Declaration of 1964 and all subsequent amendments, and all patients provided written informed consent. Patient-level data used for this analysis were de-identified. The study was approved by ethical committees of the appointed Institutions (Královské Vinohrady University Hospital, General University Hospital in Prague, St. Anne’s University Hospital Brno, University Hospital in Pilsen, and Motol University Hospital). The study was officially reported to the State Institute for Drug Control, Czech Republic, under the following identification number: 1903110001.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gkalpakiotis, S., Kojanová, M., Fialová, J. et al. Management of Moderate to Severe Plaque Psoriasis with Brodalumab in Daily Practice: Real-World Evidence from the LIBERO Study in the Czech Republic. Dermatol Ther (Heidelb) 14, 115–130 (2024). https://doi.org/10.1007/s13555-023-01066-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-01066-z