Abstract
Introduction
Evidence on treatment effectiveness in patients with psoriasis having anxiety or depressive symptoms helps shared decision-making. This single-arm, open-label, prospective study—ProLOGUE—was conducted to assess the effectiveness of brodalumab on self-assessed anxiety and depressive symptoms in Japanese patients with psoriasis.
Methods
Patients aged ≥ 18 years with plaque psoriasis without peripheral arthritis symptoms who had responded inadequately to current therapies were enrolled at 15 Japanese facilities and received brodalumab 210 mg subcutaneously.
Results
A total of 73 patients were enrolled (male, 82%; median age, 54 years). The proportion of patients without anxiety symptoms changed significantly from baseline (72.6%) to weeks 12 (88.9%, p = 0.008) and 48 (87.7%, p = 0.02); the proportion of patients without depressive symptoms did not change significantly. The Generalized Anxiety Disorder-7 score (median [quartile(Q)1–Q3], 1.0 [0.0–5.0] at baseline; 0.0 [0.0–2.0] at week 12, p = 0.008; and 0.0 [0.0–1.0] at week 48, p = 0.007) and Patient Health Questionnaire-8 score (median [Q1–Q3], 2.0 [0.0–4.0] at baseline; 1.0 [0.0–4.0] at week 12, p = 0.03; and 0.0 [0.0–2.0] at week 48, p = 0.004) significantly decreased after treatment. The median Psoriasis Area and Severity Index scores after treatment were < 1, irrespective of the presence of baseline anxiety or depressive symptoms. At week 12, the health-related quality of life was more impaired in patients with versus without baseline depressive symptoms, which largely resolved at week 48.
Conclusions
Brodalumab treatment resulted in the reduction of the levels of self-assessed anxiety and depressive symptoms in Japanese patients with psoriasis. Unlike anxiety symptoms, depressive symptoms did not resolve completely with brodalumab treatment. Patients with psoriasis having depressive symptoms may require long-term treatment.
Trial Registration
UMIN Clinical Trials Registry identifier: UMIN000027783, Japan Registry of Clinical Trials identifier: jRCTs031180037.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Brodalumab, a human anti–interleukin-17 receptor A monoclonal antibody, has demonstrated efficacy in improving the Psoriasis Area and Severity Index score and self-assessed anxiety or depressive symptoms in patients with moderate-to-severe psoriasis in a phase 3 clinical trial. |
However, disease severity of patients with psoriasis eligible for clinical trial participation may not reflect that in routine clinical practice, which warrants clinical research conducted in daily clinical settings to help shared decision-making. |
We assessed the effectiveness of brodalumab in alleviating anxiety and depressive symptoms in patients with psoriasis and the effects of these symptoms in improving the health-related quality of life of patients in daily clinical practice. |
What was learned from the study? |
Among 73 Japanese patients with psoriasis, the extent of anxiety and depressive symptoms assessed using patient-reported outcomes improved after 12 and 48 weeks of brodalumab treatment versus baseline. |
Unlike anxiety symptoms, depressive symptoms did not resolve completely, which indicates that patients with psoriasis with depressive symptoms may require longer-term treatment than those with anxiety symptoms. |
Introduction
Psoriasis is a chronic inflammatory disease that affects approximately 64.6 million individuals worldwide [1]. Published evidence suggests that patients with psoriasis commonly perceive anxiety symptoms and depressive symptoms, which can be assessed using self-administered questionnaires. In previous research, approximately 20–50% and 30% of patients with psoriasis reported anxiety symptoms and depressive symptoms, respectively [2, 3]. Apart from these symptoms, which are based on the patient’s self-assessments, anxiety disorder and depressive disorder, which are diagnosed clinically, are another burden for patients with psoriasis. Approximately 7–16% and 10% of patients with psoriasis had clinically diagnosed anxiety disorder and depressive disorder, respectively [2, 3]. High levels of anxiety and/or depressive symptoms are associated with increased levels of impairment in the health-related quality of life (HRQoL) of patients with psoriasis [4,5,6,7].
Brodalumab, a human anti–interleukin-17 receptor A monoclonal antibody, has demonstrated efficacy in improving the Psoriasis Area and Severity Index (PASI) score and self-assessed anxiety or depressive symptoms in patients with moderate-to-severe psoriasis in a phase 3 clinical trial [8]. However, evidence on its effectiveness in improving anxiety or depressive symptoms of patients with psoriasis in daily clinical settings is limited, and changes in the HRQoL of these patients following treatment initiation are not well understood. Moreover, the PASI score of patients with psoriasis who are eligible for clinical trial participation is often different from that of patients in daily clinical settings [9, 10]. Therefore, research to assist physicians in formulating treatment strategies for patients with psoriasis having anxiety or depressive symptoms in clinical practice is warranted.
The ProLOGUE study was conducted to assess the effectiveness of brodalumab in Japanese patients with psoriasis using patient-reported outcomes (PROs) [4, 10, 11]. In the baseline analysis of the ProLOGUE study, anxiety symptoms and depressive symptoms were observed in 27% and 22% of the patients, respectively, but none of the patients had clinically diagnosed anxiety disorder or depression [4]. The current analysis focused on the effectiveness of brodalumab in alleviating anxiety and depressive symptoms in patients with psoriasis and the effects of anxiety and depressive symptoms in improving their HRQoL.
Methods
Study Design and Patients
As reported previously [10, 11], this single-arm, open-label, prospective cohort study (UMIN Clinical Trials Registry identifier: UMIN000027783, Japan Registry of Clinical Trials identifier: jRCTs031180037) was conducted at 15 Japanese facilities from October 2017 to March 2020. Patients aged ≥ 18 years with plaque psoriasis without peripheral arthritis symptoms who had responded inadequately to current therapies and were eligible for self-administration of brodalumab were included in this study [10]. Patients received brodalumab 210 mg subcutaneously on day 1 and at weeks 1 and 2, followed by dosing once every 2 weeks without restrictions of concomitant or prohibited therapies. Observation was continued until week 48 of treatment [11].
This study was conducted in accordance with the Declaration of Helsinki 1964 and its later amendments. This study was first reviewed and approved by the research ethics committee of each participating facility [approval number for the representative facility (Fukuoka University): 2017M093; approval date: 2 November 2017]. Following the enforcement of the Japanese act for the conduct of clinical research funded by pharmaceutical companies in April 2018 [12], the study was reviewed and approved by the Certified Review Board of Nippon Medical School Foundation (approval number: nms2018-0601-01; approval date: 3 October 2018). All patients provided written informed consent for participation in the study [10].
Outcome Measures
The outcomes of interest of this analysis were the distribution of severity scores for anxiety and depressive symptoms at baseline and weeks 12 and 48 of brodalumab treatment, levels of anxiety and depressive symptoms at weeks 12 and 48 compared with those at baseline, and PASI and PRO scores representing the levels of HRQoL at weeks 12 and 48 in patients with and without anxiety and depressive symptoms at baseline. The PASI score was assessed by the attending physician. PRO scores were captured using an electronic PRO system before medical examination.
Anxiety symptoms during the previous 2 weeks were assessed using the Generalized Anxiety Disorder-7 (GAD-7) questionnaire (Appendix) [13]. The GAD-7 score was calculated from patients’ responses to items 1–7 of the questionnaire and classified as 0–4 (no anxiety symptoms), 5–9 (mild anxiety symptoms), 10–14 (moderate anxiety symptoms), and 15–21 (severe anxiety symptoms). A GAD-7 score of ≥ 5 was defined as having anxiety symptoms. Similarly, depressive symptoms during the previous 2 weeks were assessed using the Patient Health Questionnaire-8 (PHQ-8) (Appendix) [14, 15]. The PHQ-8 score was calculated from patients’ responses to items 1–8 of the questionnaire and classified as 0–4 (no depressive symptoms), 5–9 (mild depressive symptoms), 10–14 (moderate depressive symptoms), 15–19 (moderately severe depressive symptoms), and 20–24 (severe depressive symptoms). A PHQ-8 score of ≥ 5 was defined as having depressive symptoms. Patients’ responses to item 8 of the GAD-7 and item 9 of the PHQ-8, “If you checked off any problems, how difficult have these problems made it for you to do your work, take care of things at home, or get along with other people?” (not difficult at all, somewhat difficult, very difficult, or extremely difficult) [13,14,15], were summarized separately.
Current dermatology-specific HRQoL was measured using the Dermatology Life Quality Index (DLQI) [16]. Impairment of regular activities due to psoriasis during the previous 7 days was assessed using the Activity Impairment (AI) domain score (range, 0–100%) of the Work Productivity and Activity Impairment Questionnaire for Psoriasis (WPAI-PSO) [17]. Patients’ current health status was measured using the European Quality of Life 5-Dimension 5-Level (EQ-5D-5L) questionnaire [18]. As a generic measure of health status, the EQ-5D-5L Utility Index (UI) score (range, −0.025 to 1.000; higher scores indicate higher health utility) was calculated using the Japanese tariff [19].
Statistical Analyses
The sample size (70 patients) was determined to provide a 90% power with a two-sided significance level of 5% using a t test, assuming the mean change from baseline in the European Quality of Life 5-Dimension 3-Level of 0.2, standard deviation of 0.48, and autocorrelation of 0.2. All patients who received brodalumab were included in the analysis. The exact binomial test was used to compare the distribution of the GAD-7 (without versus with anxiety symptoms) and PHQ-8 (without versus with depressive symptoms) score categories at baseline versus those at weeks 12 and 48. The distribution of levels of difficulty in daily life due to anxiety (GAD-7 score of 0, not difficult at all, somewhat difficult, very difficult, or extremely difficult) and depressive (PHQ-8 score of 0, not difficult at all, somewhat difficult, very difficult, or extremely difficult) symptoms was analyzed in a descriptive manner at baseline and weeks 12 and 48. The GAD-7 and PHQ-8 scores at baseline were compared with those at weeks 12 and 48 using the Wilcoxon signed-rank test. PASI and PRO scores were compared between subgroups (baseline GAD-7 score of ≥ 5 versus ≤ 4 and baseline PHQ-8 score of ≥ 5 versus ≤ 4) using the Wilcoxon rank sum test.
All analyses were performed using the last observation carried forward; discontinuations up to week 12 were recorded as week 12 data, and those after week 12 were recorded as week 48 data. At baseline, week 12, and week 48, a total of 73, 73, and 69 patients, respectively, had evaluable PASI data, and complete PRO data were available for 73, 72, and 65 patients, respectively [11]. No data imputation was performed for other missing data. Statistical significance was defined as a two-tailed p-value of < 0.05. No statistical hypothesis was set, and multiplicity was not accounted for because all analyses were performed in an exploratory manner. All analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient Disposition
A total of 73 patients were enrolled in the ProLOGUE study [10]. One patient withdrew from the study before week 12 because of lack of efficacy and 16 withdrew between weeks 12 and 48 (2 for adverse events, 3 for lack of efficacy, 5 for patient withdrawal, 3 for difficulty in continuing self-injection of brodalumab, and 3 for other reasons). Consequently, 56 patients completed the study and received treatment until week 48 [11].
Patient Characteristics
The baseline characteristics of the 73 patients [60 male (82.2%)] have been reported previously [4, 10]. The median (Q1–Q3) age was 54.0 (44.0–64.0) years. Prior biologic use was reported in 29 (39.7%) patients [10]. None of the patients had a medical record of a clinically diagnosed anxiety disorder or depression after brodalumab treatment. There were no medical records suggestive of suicidal ideation or suicidal behavior during the study period.
PASI Scores and HRQoL-Related PRO Scores at Weeks 12 and 48 in Patients with and without Anxiety and Depressive Symptoms at Baseline
As reported previously, the PASI score significantly reduced from baseline to weeks 12 and 48 of brodalumab treatment [11]. There was no statistically significant difference in the PASI score at weeks 12 and 48 between patients with a GAD-7 score of ≥ 5 and those with a score of ≤ 4 at baseline. Similarly, no significant difference was observed in the PASI score between patients with a baseline PHQ-8 score of ≥ 5 and those with a score of ≤ 4. No significant difference was observed in the DLQI, WPAI-PSO AI, and EQ-5D-5L UI scores at weeks 12 and 48 between patients with a baseline GAD-7 score of ≥ 5 and those with a score of ≤ 4. At week 12, the DLQI and WPAI-PSO AI scores were significantly higher and the EQ-5D-5L UI score was significantly lower in patients with a baseline PHQ-8 score of ≥ 5 versus those with a score of ≤ 4. At week 48, the median DLQI score (1.0) and median WPAI-PSO AI score (0.0%) were similar between the PHQ-8 subgroups, and no significant difference was observed. The EQ-5D-5L UI score at week 48 was significantly lower in patients with a baseline PHQ-8 score of ≥ 5 versus those with a score of ≤ 4 (Table 1).
At baseline, there was no significant difference in the PASI score, whereas the DLQI, EQ-5D-5L UI, and WPAI-PSO AI scores were significantly different between the GAD-7 subgroups (i.e., score ≥ 5 versus ≤ 4) or between the PHQ-8 subgroups (i.e., score ≥ 5 versus ≤ 4) [4]. In addition, there was no significant difference in the EQ-5D-5L “anxiety/depression” dimension score at weeks 12 and 48 between the GAD-7 subgroups (i.e., score ≥ 5 versus ≤ 4) or between the PHQ-8 subgroups (i.e., score ≥ 5 versus ≤ 4; data not shown).
Distribution of Severity Scores for Anxiety and Depressive Symptoms at Baseline and Weeks 12 and 48 of Brodalumab Treatment
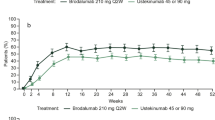
The distribution of patients without (GAD-7 score, 0–4) versus with (GAD-7 score, 5–21) anxiety symptoms significantly changed from baseline (72.6% versus 27.4%) to weeks 12 (88.9% versus 11.1%, p = 0.008) and 48 (87.7% versus 12.3%, p = 0.02) of brodalumab treatment (Fig. 1a). No significant change from baseline was observed in the distribution of patients without (PHQ-8 score, 0–4) versus with (PHQ-8 score, 5–24) depressive symptoms (78.1% versus 21.9% at baseline; 79.2% versus 20.8%, p > 0.99, at week 12; and 83.1% versus 16.9%, p = 0.34, at week 48; Fig. 1b). None of the patients had a PHQ-8 score of 20–24 (severe depressive symptoms).
Distribution of the a GAD-7 and b PHQ-8 scores at baseline and at weeks 12 and 48 in patients with psoriasis. The GAD-7 score categories of 0–4, 5–9, 10–14, and 15–21 represent no, mild, moderate, and severe anxiety symptoms, respectively. The PHQ-8 score categories of 0–4, 5–9, 10–14, and 15–19 represent no, mild, moderate, and moderately severe depressive symptoms, respectively. None of the patients showed a PHQ-8 score of 20–24 (severe depressive symptoms). The exact binomial test was used to compare the distribution of the GAD-7 (without versus with anxiety symptoms) and PHQ-8 (without versus with depressive symptoms) score categories at baseline versus weeks 12 and 48. GAD-7 Generalized Anxiety Disorder-7, PHQ-8 Patient Health Questionnaire-8
The proportion of patients having no difficulty in daily life due to anxiety symptoms (GAD-7 score of 0 or “not difficult at all” for item 8 of GAD-7) increased from baseline and exceeded 90% at weeks 12 and 48 (68.5%, 93.1%, and 90.8% at baseline, week 12, and week 48, respectively; Fig. S1a). In contrast, the proportion of patients having no difficulty in daily life due to depressive symptoms (PHQ-8 score of 0 or “not difficult at all” for item 9 of PHQ-8) showed a gradual increase from baseline to week 48 (80.8%, 88.9%, and 93.8% at baseline, week 12, and week 48, respectively; Fig. S1b).
Levels of Anxiety and Depressive Symptoms at Weeks 12 and 48 of Brodalumab Treatment Compared with Those at Baseline
In the overall population, the GAD-7 score significantly decreased from baseline [median (Q1–Q3), 1.0 (0.0–5.0)] to weeks 12 [0.0 (0.0–2.0), p = 0.008] and 48 [0.0 (0.0–1.0), p = 0.007] of brodalumab treatment (Fig. 2a). In patients with a baseline GAD-7 score of ≥ 5, the GAD-7 score significantly decreased from baseline [median (Q1–Q3), 6.5 (5.0–8.0)] to weeks 12 [1.0 (0.0–5.0), p = 0.002] and 48 [0.5 (0.0–7.0), p = 0.005]. No significant changes were observed in the GAD-7 score following brodalumab treatment in patients with a baseline GAD-7 score of ≤ 4 (Fig. 2a).
a GAD-7 and b PHQ-8 scores at baseline and at weeks 12 and 48 in patients with psoriasis. Scores were tested using the Wilcoxon signed-rank test. Top whisker represents Q3 + (1.5 × IQR) or the maximum, whichever is lower. Bottom whisker represents Q1 − (1.5 × IQR) or the minimum, whichever is higher. Top border of the box represents Q3, bottom border represents Q1, and middle (thicker) line represents the median. Anxiety symptoms + and − indicate GAD-7 scores of ≥ 5 and ≤ 4, respectively. Depressive symptoms + and − indicate PHQ-8 scores of ≥ 5 and ≤ 4, respectively. GAD-7 Generalized Anxiety Disorder-7, IQR interquartile range, PHQ-8 Patient Health Questionnaire-8, Q quartile
The PHQ-8 score decreased significantly from baseline [median (Q1–Q3), 2.0 (0.0–4.0)] to weeks 12 [1.0 (0.0–4.0), p = 0.03] and 48 [0.0 (0.0–2.0), p = 0.004] of brodalumab treatment (Fig. 2b). In patients with a baseline PHQ-8 score of ≥ 5, the PHQ-8 score decreased significantly from baseline [median (Q1–Q3), 7.5 (6.5–11.5)] to weeks 12 [5.0 (1.5–8.5), p = 0.006] and 48 [6.0 (1.0–8.0), p = 0.02], whereas no significant changes were observed in the PHQ-8 score following brodalumab treatment in patients with a baseline PHQ-8 score of ≤ 4 (Fig. 2b).
Two patients recorded a GAD-7 or PHQ-8 score of ≥ 15 following brodalumab treatment. One patient (GAD-7 score of 5 at baseline and 17 at week 12; PHQ-8 score of 3 at baseline and 15 at week 12; PASI score of 10.2 at baseline and 30.6 at week 12) reported aggravation of psoriasis as a treatment-emergent adverse event (TEAE). Another patient (PHQ-8 score of 6 at baseline and 19 at week 48; PASI score of 8.0 at baseline and 3.3 at week 48) experienced eczema and coxalgia as TEAEs. Clinically diagnosed depression or anxiety was not reported by the attending physicians as a TEAE in either of these patients.
Discussion
In the current analysis, brodalumab treatment alleviated anxiety and depressive symptoms in patients with psoriasis. Our results are consistent with the findings from clinical trials of brodalumab [8], other biologics in patients with moderate-to-severe psoriasis [20,21,22,23], and research conducted outside Japan that did not include patients treated with brodalumab [24,25,26]. In our cohort, two patients experienced aggravation of anxiety and depressive symptoms (GAD-7 or PHQ-8 score of ≥ 15) after initiation of brodalumab treatment. TEAEs reported in these patients (aggravation of psoriasis and eczema) suggest an influence of skin lesion severity on the levels of anxiety and depressive symptoms in patients with psoriasis [27]. In line with previous literature [28,29,30], there were no medical records suggestive of suicidal ideation or behavior among patients treated with brodalumab. Of note, the Japanese package insert of brodalumab states that administration of brodalumab to patients with current or past history of depression or depressive state or those with a history of suicidal ideation and/or suicide attempts requires caution [31]. Accordingly, only individuals who were deemed appropriate for brodalumab use were enrolled in the current study. In addition, the Japanese guidance for the use of biologics for psoriasis does not mandate the monitoring of suicidal ideation and behavior using the Columbia-Suicide Severity Rating Scale for patients treated with brodalumab [32, 33]; thus, patient screening or study assessment using this scale was not performed.
Although neither anxiety nor depressive symptoms correlated with the PASI score in the baseline analysis of the ProLOGUE study [4], the proportion of patients without anxiety symptoms significantly increased with brodalumab treatment; the proportion of patients without depressive symptoms tended to increase numerically toward week 48 without any significant difference. In addition, our results indicated greater negative effects of depressive symptoms than those of anxiety symptoms on the HRQoL of patients with psoriasis at week 12, which largely resolved at week 48. To improve the HRQoL of patients with depressive symptoms in the long term, sustained improvement in skin lesions, a good doctor–patient relationship to alleviate depressive symptoms, and improvement in other psoriasis-related symptoms may be required.
The difference in the effects of anxiety and depressive symptoms on disease severity and treatment outcomes in patients with psoriasis has been discussed in published studies [20, 34,35,36]. Although both anxiety and depressive symptoms are characterized by repetitive negative thinking, the former is mediated by potential threats, uncertainties, and risks in the future, whereas the latter is a reflection of past-oriented mental perseveration on the causes and consequences of one’s distress [37]. Moreover, elevation in proinflammatory cytokines has been suggested as a factor common to the progression of psoriasis and depression [38,39,40]. Results of the current analysis can be attributed to these psychological and biological factors, which should be explored in future large-scale studies.
This analysis has some limitations. First, analysis of the difference in treatment outcomes between patients with anxiety symptoms only, depressive symptoms only, or both symptoms at baseline was not feasible because of the small sample size for subgroup analyses. Second, the generalizability of the current results to patients outside Japan may be limited, although the proportion of patients with psoriasis having anxiety or depressive symptoms is not largely different between Japan and other countries [2,3,4]. Third, the generalizability of the current results to other biologics may need further investigation, as reports of similar studies remain limited [20]. Fourth, analysis by sex was not feasible because of the limited number of women enrolled, although evidence suggests an association between female sex and anxiety and depression scores in patients with psoriasis [6, 7, 36]. Lastly, it is not clear whether patients had a clinical diagnosis of depression or anxiety or received any interventions to manage anxiety or depressive symptoms outside the dermatology departments.
Conclusions
Brodalumab treatment resulted in the reduction of the levels of anxiety and depressive symptoms in Japanese patients with psoriasis. Our results suggest that anxiety symptoms in patients with psoriasis improve with improvement in skin lesions and that patients with depressive symptoms have a longer duration of impaired HRQoL than those with anxiety symptoms. Long-term monitoring for changes in HRQoL and treatment outcomes may be required for patients with psoriasis who have depressive symptoms at the time of initiating biologics.
References
AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis—comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol. 2020;59:566–71. https://doi.org/10.1111/ijd.14864.
Fleming P, Bai JW, Pratt M, Sibbald C, Lynde C, Gulliver WP. The prevalence of anxiety in patients with psoriasis: a systematic review of observational studies and clinical trials. J Eur Acad Dermatol Venereol. 2017;31:798–807. https://doi.org/10.1111/jdv.13891.
Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542–51. https://doi.org/10.1038/jid.2013.508.
Ohata C, Kanai Y, Murotani K, Kitabayashi H, Imafuku S. Difference in health-related quality of life between anxiety and depressive symptoms in Japanese patients with plaque psoriasis: the ProLOGUE study. J Eur Acad Dermatol Venereol. 2022;36:e57–9. https://doi.org/10.1111/jdv.17621.
Bakar RS, Jaapar SZS, Azmi AF, Aun YC. Depression and anxiety among patients with psoriasis: a correlation with quality of life and associated factors. J Taibah Univ Med Sci. 2021;16:491–6. https://doi.org/10.1016/j.jtumed.2021.02.008.
Martínez-Ortega JM, Nogueras P, Muñoz-Negro JE, Gutiérrez-Rojas L, González-Domenech P, Gurpegui M. Quality of life, anxiety and depressive symptoms in patients with psoriasis: a case-control study. J Psychosom Res. 2019;124:109780. https://doi.org/10.1016/j.jpsychores.2019.109780.
Pollo CF, Miot HA, Matos TDS, et al. Prevalence and factors associated with depression and anxiety in patients with psoriasis. J Clin Nurs. 2021;30:572–80. https://doi.org/10.1111/jocn.15577.
Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273–86. https://doi.org/10.1111/bjd.14493.
Masson Regnault M, Castañeda-Sanabria J, Diep Tran MHT, et al. Users of biologics in clinical practice: would they be eligible for phase III clinical studies? Cohort study in the French Psoriasis Registry PSOBIOTEQ. J Eur Acad Dermatol Venereol. 2020;34:293–300. https://doi.org/10.1111/jdv.15878.
Imafuku S, Kanai Y, Murotani K, et al. Utility of the Dermatology Life Quality Index at initiation or switching of biologics in real-life Japanese patients with plaque psoriasis: results from the ProLOGUE study. J Dermatol Sci. 2021;101:185–93. https://doi.org/10.1016/j.jdermsci.2021.01.002.
Imafuku S, Ohata C, Okubo Y, et al. Effectiveness of brodalumab in achieving treatment satisfaction for patients with plaque psoriasis: the ProLOGUE study. J Dermatol Sci. 2022;105:176–84. https://doi.org/10.1016/j.jdermsci.2022.02.007.
Nakamura K, Shibata T. Regulatory changes after the enforcement of the new Clinical Trials Act in Japan. Jpn J Clin Oncol. 2020;50:399–404. https://doi.org/10.1093/jjco/hyaa028.
Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–7. https://doi.org/10.1001/archinte.166.10.1092.
Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. https://doi.org/10.1016/j.jad.2008.06.026.
Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509–15. https://doi.org/10.3928/0048-5713-20020901-06.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. https://doi.org/10.1111/j.1365-2230.1994.tb01167.x.
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–65. https://doi.org/10.2165/00019053-199304050-00006.
Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36. https://doi.org/10.1007/s11136-011-9903-x.
Ikeda S, Shiroiwa T, Igarashi A, et al. Developing a Japanese version of the EQ-5D-5L value set. J Natl Inst Public Health. 2015;64:47–55.
Talamonti M, Malara G, Natalini Y, et al. Secukinumab improves patient perception of anxiety and depression in patients with moderate to severe psoriasis: a post hoc analysis of the SUPREME study. Acta Derm Venereol. 2021;101:adv00422. https://doi.org/10.2340/00015555-3712.
Thaçi D, Soliman AM, Eyerich K, et al. Patient-reported outcomes with risankizumab versus fumaric acid esters in systemic therapy-naïve patients with moderate to severe plaque psoriasis: a phase 3 clinical trial. J Eur Acad Dermatol Venereol. 2021;35:1686–91. https://doi.org/10.1111/jdv.17109.
Augustin M, Lambert J, Zema C, et al. Effect of risankizumab on patient-reported outcomes in moderate to severe psoriasis: the UltIMMa-1 and UltIMMa-2 randomized clinical trials. JAMA Dermatol. 2020;156:1344–53. https://doi.org/10.1001/jamadermatol.2020.3617.
Gordon KB, Armstrong AW, Han C, et al. Anxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the Phase 3 VOYAGE 2 study. J Eur Acad Dermatol Venereol. 2018;32:1940–9. https://doi.org/10.1111/jdv.15012.
Leman J, Walton S, Layton AM, et al. The real world impact of adalimumab on quality of life and the physical and psychological effects of moderate-to-severe psoriasis: a UK prospective, multicenter, observational study. J Dermatolog Treat. 2020;31:213–21. https://doi.org/10.1080/09546634.2019.1592096.
Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70–80. https://doi.org/10.1016/j.jaad.2017.08.051.
Salame N, Ehsani-Chimeh N, Armstrong AW. Comparison of mental health outcomes among adults with psoriasis on biologic versus oral therapies: a population-based study. J Dermatolog Treat. 2019;30:135–40. https://doi.org/10.1080/09546634.2018.1476654.
Tribó MJ, Turroja M, Castaño-Vinyals G, et al. Patients with moderate to severe psoriasis associate with higher risk of depression and anxiety symptoms: results of a multivariate study of 300 Spanish individuals with psoriasis. Acta Derm Venereol. 2019;99:417–22. https://doi.org/10.2340/00015555-3114.
Koo J, Ho RS, Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis. 2019;104:361–5.
Iznardo H, Puig L. The safety of brodalumab for the treatment of psoriasis. Expert Opin Drug Saf. 2020;19:365–72. https://doi.org/10.1080/14740338.2020.1730326.
Rivera-Oyola R, Stanger R, Litchman GH, et al. The use of brodalumab in three patients with psoriasis and psychiatric comorbidities. J Clin Aesthet Dermatol. 2020;13:44–8.
Kyowa Kirin. LUMICEF® Subcutaneous Injection 210 mg Syringe. Package insert version 4, revised June 2022. In Japanese.
Saeki H, Terui T, Morita A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 version). J Dermatol. 2020;47:201–22.
Saeki H, Mabuchi T, Asahina A, et al. English version of Japanese guidance for use of biologics for psoriasis (the 2022 version). J Dermatol. 2023;50:e41–68.
Thaçi D, Kimball A, Foley P, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, improves patient-reported outcomes in the treatment of moderate to severe psoriasis: results of two phase III randomized, controlled trials. J Eur Acad Dermatol Venereol. 2017;31:498–506. https://doi.org/10.1111/jdv.13918.
Łakuta P, Przybyła-Basista H. Toward a better understanding of social anxiety and depression in psoriasis patients: the role of determinants, mediators, and moderators. J Psychosom Res. 2017;94:32–8. https://doi.org/10.1016/j.jpsychores.2017.01.007.
Yang A, Xin X, Yang W, et al. Etanercept reduces anxiety and depression in psoriasis patients, and sustained depression correlates with reduced therapeutic response to etanercept. Ann Dermatol Venereol. 2019;146:363–71. https://doi.org/10.1016/j.annder.2019.03.002.
Gustavson DE, du Pont A, Whisman MA, Miyake A. Evidence for transdiagnostic repetitive negative thinking and its association with rumination, worry, and depression and anxiety symptoms: a commonality analysis. Collabra Psychol. 2018;4:13. https://doi.org/10.1525/collabra.128.
González-Parra S, Daudén E. Psoriasis and depression: the role of inflammation. Actas Dermosifiliogr (Engl Ed). 2019;110:12–9. https://doi.org/10.1016/j.ad.2018.05.009.
Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613–20. https://doi.org/10.1007/s40257-017-0279-8.
Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999–2009. https://doi.org/10.1111/jdv.14460.
Acknowledgements
We thank the participants of the study.
Funding
This study was funded by Kyowa Kirin Co., Ltd. Employees of Kyowa Kirin Co., Ltd. were involved in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication. The study sponsor is also funding the journal’s Rapid Service Fees.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Mami Hirano, MS, of Cactus Life Sciences (part of Cactus Communications) and funded by Kyowa Kirin Co., Ltd. Statistical analysis support based on authors’ detailed directions, in the form of statistical analysis plan preparation, analysis program creation, and analysis data quality control, was provided by Masashi Suzuki of I’cros Co., Ltd., and funded by Kyowa Kirin Co., Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Chika Ohata, Yasumasa Kanai, Kenta Murotani; Methodology: Yasumasa Kanai, Kenta Murotani, Takanobu Nomura; Software: Kenta Murotani; Formal analysis: Kenta Murotani; Investigation: Chika Ohata, Fumikazu Yamazaki, Hidetoshi Takahashi, Yayoi Tada, Tomotaka Mabuchi, Yoko Mizutani, Shinichi Imafuku; Resources: Chika Ohata, Kenta Murotani, Fumikazu Yamazaki, Hidetoshi Takahashi, Yayoi Tada, Tomotaka Mabuchi, Yoko Mizutani, Shinichi Imafuku; Data Curation: Kenta Murotani, Shinichi Imafuku; Writing - Review & Editing: all authors; Visualization: Chika Ohata, Yasumasa Kanai, Kenta Murotani, Takanobu Nomura, Shinichi Imafuku; Supervision: Shinichi Imafuku; Project administration: Yasumasa Kanai, Shinichi Imafuku; Funding acquisition: Shinichi Imafuku
Prior Presentation
The work reported in the current manuscript was partly presented at the 2020 American Academy of Dermatology Annual Meeting (ePoster# 14004).
Disclosures
C. Ohata currently belongs to Department of Dermatology, Osaka General Medical Center, and reports receiving grants from Kyowa Kirin during the conduct of the study; grants, personal fees, and nonfinancial support from Kyowa Kirin, Eisai, Eli Lilly Japan, Novartis Pharma, Celgene, and Taiho Pharmaceutical outside the submitted work; personal fees and nonfinancial support from AbbVie, Janssen Pharmaceuticals, MSD, and UCB Japan outside the submitted work; and personal fees from Hisamitsu Pharmaceutical outside the submitted work. Y. Kanai and T. Nomura are employees of Kyowa Kirin. K. Murotani reports grants from Kyowa Kirin during the conduct of the study. F. Yamazaki reports grants from Kyowa Kirin during the conduct of the study; grants, personal fees, and nonfinancial support from Kyowa Kirin, Mitsubishi Tanabe Pharma, AbbVie, Eisai, Eli Lilly, Torii Pharmaceutical, Taiho Pharmaceutical, Maruho, and Novartis Pharma outside the submitted work; and personal fees and nonfinancial support from Celgene, Janssen Pharmaceuticals, and UCB Japan outside the submitted work. H. Takahashi reports grants from Kyowa Kirin during the study period. Y. Tada reports grants from Kyowa Kirin during the study period; grants, personal fees, and nonfinancial support from Kyowa Kirin, Eli Lilly Japan, AbbVie, Maruho, Celgene, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma, Novartis Pharma, Sanofi, UCB Japan, Torii Pharmaceutical, LEO Pharma, Eisai, Kaken Pharmaceutical, Pfizer, Ushio, Meiji Seika Pharma, Nippon Boehringer Ingelheim, JIMRO, Bristol Myers Squibb, and TOKIWA Pharmaceutical outside the submitted work; grants and nonfinancial support from Kanebo Cosmetics, MSD, Ono Pharmaceutical, Pola Pharma, Nihon Pharmaceutical, Smith & Nephew, and Sato Pharmaceutical outside the submitted work; personal fees and nonfinancial support from Janssen Pharmaceuticals outside the submitted work; grants from Japan Blood Products Organization, Mochida Healthcare, Oshimatsubaki, and Shionogi outside the submitted work; and personal fees from Chugai Pharmaceutical outside the submitted work. T. Mabuchi reports grants from Kyowa Kirin during the conduct of the study; grants, personal fees, and nonfinancial support from Kyowa Kirin, Maruho, Taiho Pharmaceutical, and Torii Pharmaceutical outside the submitted work; grants from LEO Pharma outside the submitted work; and personal fees and nonfinancial support from Janssen Pharmaceuticals, Celgene, Novartis Pharma, and Eli Lilly Japan outside the submitted work. Y. Mizutani reports grants from Kyowa Kirin during the conduct of the study; grants, personal fees, and nonfinancial support from Novartis Pharma, Maruho, and Eli Lilly Japan outside the submitted work; and grants from Taiho Pharmaceutical, Sanofi, Mitsubishi Tanabe Pharma, Kyowa Kirin, Sun Pharma, Kaken Pharmaceutical, and Japan Blood Products Organization outside the submitted work. S. Imafuku reports grants from Kyowa Kirin during the conduct of the study; grants and personal fees from AbbVie, Eisai, Kaken Pharmaceutical, Kyowa Kirin, Sato Pharmaceutical, Sanofi, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma, Tsumura, Torii Pharmaceutical, Nippon Zoki Pharmaceutical, Novartis Pharma, Maruho, and LEO Pharma outside the submitted work; grants from Pola Pharma outside the submitted work; and personal fees from Astellas, Eli Lilly Japan, MSD, Otsuka, Ono Pharmaceutical, Sun Pharma, GSK, JIMRO, Celgene, Daiichi Sankyo, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Japan Blood Products Organization, Pfizer, Bristol Myers Squibb, Meiji Seika Pharma, Janssen Pharmaceuticals, and UCB Japan outside the submitted work.
Compliance with Ethics Guidelines
This study was conducted in accordance with the Declaration of Helsinki 1964 and its later amendments. This study was first reviewed and approved by the research ethics committee of each participating facility (approval number for the representative facility [Fukuoka University]: 2017M093; approval date: November 2, 2017). Following the enforcement of the Japanese act for the conduct of clinical research funded by pharmaceutical companies in April 2018, the study was reviewed and approved by the Certified Review Board of Nippon Medical School Foundation (approval number: nms2018-0601-01; approval date: October 3, 2018). All patients provided written informed consent for participation in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because study enrollment began before January 1, 2019, and the informed consent did not specify data sharing for research purposes.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ohata, C., Kanai, Y., Murotani, K. et al. Effectiveness of Long-Term Treatment with Brodalumab on Anxiety or Depressive Symptoms in Japanese Patients with Psoriasis: The ProLOGUE Study. Dermatol Ther (Heidelb) 13, 1039–1052 (2023). https://doi.org/10.1007/s13555-023-00909-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00909-z