Abstract
Introduction
Early psoriatic arthritis (PsA) diagnosis is critical to prescribe timely treatment to prevent the irreversible joint damage and the many other problems that patients with PsA experience. This retrospective study aimed to highlight the benefits of a Rheuma–Derma Clinic focused on the early diagnosis and prompt treatment of PsA with a shared approach among Italian psoriasis patients. Diagnosing PsA early is the main goal to reduce joint damage and disability in the patients affected. Studies describing the results of rheuma–derma clinics aimed to reach this goal emerged in the last decade. This study presents limitations and advantages typical of retrospective designs.
Methods
A Rheuma–Derma Clinic was created in 2017 at the Rheumatology Department of the Hospital Policlinico Gaspare Rodolico of the University of Catania in San Marco, Italy. This study compared the number of patients under disease-modifying antirheumatic treatment 5 years before and after the joint clinic was created. A rheumatologist and dermatologist simultaneously assessed patients with psoriasis and/or PsA to obtain a rapid multidisciplinary diagnostic approach and a shared therapeutic strategy. In addition, demographic, clinical, and clinimetrics data were collected.
Results
The number of patients with PsA receiving biological disease-modifying antirheumatic drugs increased 47% (from 255 to 374 patients) before and after the joint clinic was implemented. Likewise, those receiving conventional synthetic disease-modifying antirheumatic drugs increased by 47% (from 367 to 539) as well. Additionally, for all the clinimetrics evaluated (DAS28, HAQ, BASDAI, DAPSA, PASI, PGA), there was an improvement over the 12 months under the Rheuma–Derma Clinic care. The measures that improved the most were DAPSA, PGA, PASI, and BASDAI.
Conclusion
The implementation of the Rheuma–Derma Clinic was associated with an increase in the number of patients diagnosed, the number of patients with PsA receiving DMARD treatments, and improvements in clinimetrics among the study participants.
Plain language summary
Psoriatic arthritis needs to be detected early to treat it correctly and for its consequences in patients with psoriasis to be prevented. This study was carried out to find out if shared clinics where rheumatologists and dermatologists care for patients together helped to control psoriatic arthritis better and improve the life of the patients affected by it. The shared clinic was created in 2017 at the Rheumatology Department of the Hospital Policlinico Gaspare Rodolico of the University of Catania in San Marco, Italy. The study collected information about the number of patients diagnosed with psoriasis and psoriatic arthritis, the number of patients receiving treatments, and the results of quality of life and other standard tests. During the time that the joint clinic was in place, the number of patients receiving treatments specific for diseases such as psoriatic arthritis increased from 622 (before the clinic was started) to 913. The patients treated in this clinic also had improvements in their quality of life and other aspects. This study found that a clinic where rheumatologists and dermatologists work together to care for patients can increase the number of patients treated with drugs that are specific for their disease and improve their overall health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Early detection and treatment of PsA are critical to diminish the possibility of permanent joint damage, disability, and more comorbidities in patients with psoriasis. |
Comprehensive evaluations performed jointly by rheumatologists and dermatologists can help improve the health outcomes of patients with PsA. |
This retrospective study aimed to highlight the benefits of a Rheuma–Derma Clinic focused on the early diagnosis and prompt treatment of PsA with a shared approach among Italian patients with psoriasis. |
What was learned from the study? |
The number of patients with PsA receiving biological disease-modifying antirheumatic drugs increased 47% (from 255 to 374 patients) before and after the joint clinic was implemented. In addition, the number of patients receiving conventional synthetic disease-modifying antirheumatic drug also increased 47% (from 367 to 539). |
The Rheuma–Derma Clinic at the Hospital Policlinico Gaspare Rodolico of the University of Catania was associated with an increase in the number of patients diagnosed, the number of patients with PsA receiving appropriate treatments, and improvements in clinimetrics among the patients involved. |
Introduction
Psoriatic arthritis (PsA) is an inflammatory joint disease that affects patients with psoriasis. It may involve other organs besides the musculoskeletal system and the skin, needing a multidisciplinary approach that should implicitly involve different medical specialties. Many recent works in the literature have underlined the importance of a combined approach between the dermatologist and rheumatologist, primarily because the percentage of undiagnosed PsA in patients with psoriasis is still high [1]. Concretely, PsA affects between 20% and 30% of individuals with psoriasis [2, 3]. According to a systematic review and meta-analysis that included 266 studies and close to 1,000,000 patients, 19.7% of patients with psoriasis also had PsA [95% confidence interval (CI) 18.5–20.9%], and this increased to 24.6% among patients with moderate to severe psoriasis [2]. When rheumatologists assessed the possibility of PsA, its prevalence was 30% (95% CI 27–33%). Notably, 41% of these cases had not been previously diagnosed with PsA [3]. This indicates that early rheumatologic evaluations represent a significant unmet need among patients with psoriasis. In fact, earlier detection and treatment of PsA is critical to diminish the possibility of permanent joint damage, disability, and more comorbidities in patients with psoriasis [3].
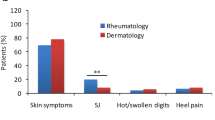
Since psoriasis usually precedes the onset of PsA, dermatologists should prioritize the early identification of its signs and symptoms. Patients with specific psoriasis phenotypes (scalp, inverse, and nail psoriasis) and risk factors such as obesity, family history of PsA, and severe psoriasis should receive particular attention since they have greater chances of developing PsA [4]. Additionally, as shown by a retrospective analysis of data from the Institute of Applied Health Research Berlin, patients with psoriasis who experienced acute rheumatism or pain in unspecific joints are at an increased risk of developing PsA as well [5]. Dermatologists should particularly assess complaints of joint pain, stiffness, and swelling, with particular attention to their onset, duration, and relationship to exercise. The physical examination should include at least the affected hands, feet, and joints to investigate possible axial and peripheral joint involvement. Any patient with disabling joint symptoms or symptoms unresponsive to nonsteroidal anti-inflammatory drugs should be referred to a rheumatologist. At the same time, rheumatologists should work with dermatologists to ensure that skin symptoms are addressed, directing them toward the possible addition of topical therapies (phototherapy and combinations of corticosteroids and vitamin D derivatives) when a systemic treatment is already in place but it does not entirely control skin manifestations. Furthermore, in some cases, PsA treatment with tumor necrosis factor (TNF) inhibitors and systemic glucocorticoids can lead to skin complications such as an exacerbation of psoriasis (paradoxical psoriasis) [4].
Patients with PsA also experience a heavier psychosocial burden and require prolonged follow-up and treatments that have reached higher complexity through the availability of new assessment tools and treatments. Therefore, a multidisciplinary and integrated approach to diagnosis and treatment involving, at a minimum, rheumatologists and dermatologists may represent a more effective and comprehensive approach to care for these patients [6]. Furthermore, the better management of these patients is expected to reduce their risk of joint damage, disability, and comorbidities [6]. As Helliwell, et al. pointed out, multidisciplinary clinics have already been implemented in the USA (Harvard University, The Cleveland Clinic, and Stanford University) and Europe (the United Kingdom, Germany, Italy, and Spain) [7]. Different models for combined rheumatologic and dermatologic care exist. For instance, they may be in a single institution or be part of multicentric networks such as the Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network (PPACMAN) [1, 8, 9].
The study presented here aims to highlight the benefits of a Rheuma–Derma Clinic focused on the early diagnosis and prompt treatment of PsA with a shared approach among Italian patients with psoriasis.
Methods
A Rheuma–Derma Clinic was created in 2017 at the Rheumatology Department of the Hospital Policlinico Gaspare Rodolico of the University of Catania in San Marco, Italy. This retrospective observational study was conducted to compare PsA data 5 years before and after this Rheuma–Derma Clinic was implemented; therefore, from 2012 to 2016 compared with 2017 to 2021.
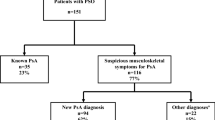
Patients with psoriasis complaining of joint symptoms as well as rheumatologic patients with cutaneous involvement were included in the study. A total of 906 patients with PsA in accordance with the ClASsification for Psoriatic ARthritis (CASPAR) Criteria [3] were enrolled in this study. Patients were identified from an internal database at the Rheumatology Department of the Hospital Policlinico Gaspare Rodolico of the University of Catania in San Marco, Italy. The local prevalence of PsA is in line with literature [2].
The combined Rheuma–Derma Clinic consisted of rheumatologists and dermatologists working together and approaching all patients with standardized diagnostic tools and therapeutic protocols. The shared rheuma–derma visit is intended to examine each patient simultaneously, effectively evaluating their clinical history, comorbidities, weight, joint affection pattern (axial/peripheral), family history of psoriasis or PsA, active psoriasis, and nail, ocular, or gastrointestinal involvement. This standardized scheme focused on the global medical history of each patient to select individualized treatments using a combined approach by a united group of specialists.
The data collected were demographic and clinical characteristics (joint affection patterns, axial/peripheral) [10, 11], clinimetrics [Disease Activity Score 28 (DAS 28), Health Assessment Questionnaire (HAQ), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Disease Activity Index for Psoriatic Arthritis (DAPSA), Psoriasis Area Severity Index (PASI), Physician Global Assessment (PGA)], family history of psoriasis or PsA, body mass index (BMI), psoriasis history, comorbidities [12], and Charlson Comorbidity Index (CCI) [13], gastrointestinal and ophthalmic involvement, and previous and current treatments with conventional synthetic disease-modifying antirheumatic drug (csDMARDS) and/or biological disease-modifying antirheumatic drugs (bDMARDs).
The CCI assesses the level of comorbidities patients have by considering the number and severity of predefined comorbid conditions as well as the age of patients. It provides a weighted score in a range from 0 to 8, where higher values indicate more severe comorbidities that can be used to predict short-term and long-term outcomes such as loss of function, hospital length of stay, and mortality rates [13].
Data from the patients’ records were transferred to a database and descriptive statistics using mean and standard deviation (SD) were conducted. The ethics committee of the University of Catania approval is 170/11-02-2013. The study was conducted in compliance with the Helsinki Declaration of 1964 and its later amendments. All subjects provided informed consent to participate in the study.
Results
The patients included had a median age of 57 years, and almost three-quarters of them were female (70%). Over half (56%) of the patients that attended the Rheuma–Derma Clinic had a normal BMI [14], and a third of them were overweight (31%); only 11% of them presented an obesity grade I (Table 1). In addition, a relatively small number of patients had PsA family history (24%).
In terms of clinical characteristics, the joints affected were mostly peripheral (69%). About one in ten (11%) cases had axial joints affected, and two in ten (20%) cases had a mixed pattern of joint involvement. Three-quarters (75%) of the patients had moderate psoriasis, while one-fifth (19%) had severe psoriasis, and a few (6%) had mild disease.
During the Rheuma–Derma Clinic, psoriasis was diagnosed in 41% of patients. In 80% of these patients, the skin disease preceded the articular symptoms, while 20% of the patients developed psoriasis after their arthritis onset.
As shown in Table 2, during the period preceding the implementation of the joint Rheuma–Derma Clinic (2012–2016), the Rheumatology Department’s records showed that 255 patients with PsA were receiving bDMARDs ± csDMARDs. That number increased to 374 patients (an increase of 47%) after the Rheuma–Derma Clinic (2017–2021) was implemented. Likewise, 367 patients with PsA received csDMARDs before the Rheuma–Derma Clinic was implemented, and 539 patients (an increase of 47%) did so after the shared clinic was implemented. Globally, the number of patients with PsA receiving either class of DMARDs went from 622 to 913 (a 47% increase).
In the group analyzed, 539 patients were treated with csDMARDs; of these, three-quarters (62%) received methotrexate, almost a third (31%) salazopyrine, and only 7% received cyclosporine. A total of 374 patients received a bDMARD alone or in addition to csDMARDs; of these, most of the patients (49%) were treated with tumor necrosis factor-alpha (TNF-α) inhibitors, a third were treated with interleukin-17 inhibitors (25% secukinumab, 5% ixekizumab), and one-fifth were treated with interleukin-12 and 23 inhibitors (20% ustekinumab). Some patients received other bDMARDs as shown in Table 1.
Regarding CCI assessed in the present study, most patients (27%) had a score of 2, while only 0.5% of the population had a score of 8. The most frequent comorbidities registered in the present cohort were hypertension (25%) and dyslipidaemia (15%). Nail involvement was observed only in 8% of patients, followed by uveitis (7%) and gastrointestinal involvement (3%).
Discussion
In the period between 2017 and 2021, there was a 47% increase in patients with PsA that were treated with bDMARDs, csDMARDs, or both. Furthermore, several clinimetrics measures improved for these patients while they were under the care of the join Rheuma–Derma Clinic at the Hospital Policlinico Gaspare Rodolico of the University of Catania (Table 3).
Early diagnosis of PsA is the main goal to reduce joint damage and disability in patients affected by this condition. Studies describing and/or sharing the results of rheuma–derma clinics aiming to reach this goal have emerged in the last decade. Soleymani, et al. described 20 combined rheumatology–dermatology clinics in the United States, including Stanford, New York University, Harvard, University of Pennsylvania, and the National Psoriasis Foundation in Oregon. These combined clinics take place between once a week and once a month and are implemented in different modalities (specialists provide care simultaneously or in staggered evaluations on the same day, or virtually with a communication relationship). Overall, both types of specialists reported that earlier and more accurate diagnoses achieved prompt, timely interventions, and improved outcomes. The challenges they experienced are mostly related to scheduling, obtaining institutional support, billing, and the time they required [15]. Luchetti, et al. described a dermo–rheumatologic clinic in Italy where 482 consecutive patients were assessed simultaneously by corresponding specialists from September 2015 to September 2017. They found that the tight clinical cooperation facilitated optimal PsA management and the earlier prescription of bDMARDs. Since no difference was detected in the clinical outcomes of the patients that received csDMARDs or bDMARDs, the investigators propose that the outcome improvements could be related to the optimal management that was provided. Although this project did not involve a control group of patients managed conventionally, it is widely known that the standard approach is inadequate for these patients [1]. Urruticoechea-Arana, et al. performed a retrospective records review of 112 patients with PsA who attended a multidisciplinary clinic in Spain for almost 3 years. The reasons for attending the clinic were equally divided between diagnostic doubts and therapeutic difficulties. Almost 60% of the patients met diagnostic criteria for PsA and 11% of those of a different inflammatory disease. Their topical and systemic treatments were changed in 55.4% and 42% of the cases, respectively. The patients were highly satisfied and felt that their condition was better controlled by attending the multidisciplinary clinic. Collaboration between the rheumatology and dermatology departments improved [16]. Lastly, a systematic review by Cobo-Ibañez, et al. described the outcomes of patients that were evaluated weekly or monthly in multidisciplinary clinics. It included two case series and one descriptive study with a collective total of 506 patients with psoriasis and PsA. Although the evidence was lacking, it suggests the multidisciplinary consultations improved clinical manifestations of the disease after changing treatments compared with the usual approach, and patients were very satisfied. The disadvantage was that the waiting times were longer [17].
Furthermore, a screening tool such as the Psoriasis Epidemiology Screening Tool (PEST) for PsA would be useful to both general practitioners and dermatologists and would help identify patients needing further early evaluation by a rheumatologist [18]
Although retrospective studies such as the one reported here allow investigators to collect data covering a long period of time, are relatively simple, and inexpensive to implement, they present limitations that should be considered. For instance, the use of medical records not collected for research purposes may have inherent problems related to missing information. Also, there may have been many sources of bias (selection, lost to follow-up, recall), and no adjustments were performed to account for potential confounders.
Conclusion
Despite the lack of generalizability of the results of this study, they contribute to the medical literature on the subject. This experience found that the implementation of the Rheuma–Derma Clinic was associated with an increase in the number of patients with PsA under DMARD treatments and improvements in clinimetrics among the study participants. However, well-designed prospective studies are necessary to provide generalizable data on this subject.
References
Luchetti MM, Benfaremo D, Campanati A, et al. Clinical outcomes and feasibility of the multidisciplinary management of patients with psoriatic arthritis: two-year clinical experience of a dermo-rheumatologic clinic. Clin Rheumatol. 2018;37(10):2741–9.
Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251-65.e19.
Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–35.
Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. 2019;15(3):153–66.
Rech J, Sticherling M, Stoessel D, Biermann MHC, Häberle BM, Reinhardt M. Psoriatic arthritis epidemiology, comorbid disease profiles and risk factors: results from a claims database analysis. Rheumatol Adv Pract. 2020. https://doi.org/10.1093/rap/rkaa033.
Visalli E, Crispino N, Foti R. Multidisciplinary management of psoriatic arthritis: the benefits of a comprehensive approach. Adv Ther. 2019;36(4):806–16.
Helliwell P, Coates L, Chandran V, et al. Qualifying unmet needs and improving standards of care in psoriatic arthritis. Arthritis Care Res (Hoboken). 2014;66(12):1759–66.
Okhovat JP, Ogdie A, Reddy SM, Rosen CF, Scher JU, Merola JF. Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network Consortium (PPACMAN) Survey: benefits and challenges of combined rheumatology-dermatology clinics. J Rheumatol. 2017;44(5):693–4.
Velez NF, Wei-Passanese EX, Husni ME, Mody EA, Qureshi AA. Management of psoriasis and psoriatic arthritis in a combined dermatology and rheumatology clinic. Arch Dermatol Res. 2012;304(1):7–13.
Jadon DR, Sengupta R, Nightingale A, et al. Axial disease in Psoriatic Arthritis Study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis. 2017;76(4):701–7.
Chandran V, Stecher L, Farewell V, Gladman DD. Patterns of peripheral joint involvement in psoriatic arthritis-symmetric, ray and/or row? Semin Arthritis Rheum. 2018;48(3):430–5.
Sultana A, Bhuiyan SI, Mahmud MM, Siddique RU, Shawkat SM, Nandi AK. Comorbidities in patients with psoriasis. Mymensingh Med J. 2019;28(4):894–9.
Brusselaers N, Lagergren J. The Charlson Comorbidity Index in registry-based research. Methods Inf Med. 2017;56(5):401–6.
Kumthekar A, Ogdie A. Obesity and psoriatic arthritis: a narrative review. Rheumatol Ther. 2020;7(3):447–56.
Soleymani T, Reddy SM, Cohen JM, Neimann AL. Early recognition and treatment heralds optimal outcomes: the benefits of combined rheumatology-dermatology clinics and integrative care of psoriasis and psoriatic arthritis patients. Curr Rheumatol Rep. 2017;20(1):1.
Urruticoechea-Arana A, Serra Torres M, Hergueta Diaz M, et al. Experience and satisfaction with a multidisciplinary care unit for patients with psoriasis an psoriatic arthritis. Reumatol Clin (Engl Ed). 2019;15(4):237–41.
Cobo-Ibáñez T, Villaverde V, Seoane-Mato D, et al. Multidisciplinary dermatology-rheumatology management for patients with moderate-to-severe psoriasis and psoriatic arthritis: a systematic review. Rheumatol Int. 2016;36(2):221–9.
Helliwell PS. Psoriasis Epidemiology Screening Tool (PEST): a report from the GRAPPA 2009 annual meeting. J Rheumatol. 2011;38(3):551–2.
Acknowledgements
The study investigators thank the patients of this study for their involvement in the study.
Funding
The journal’s Rapid Service Fee was funded by Novartis Farma S.p.A.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by María Carolina Rojido (Medical Writing Consultant). Proofreading and editing assistance provided by Laura C Collada Ali (Medical Writing Consultant). This was funded by Novartis Farma S.p.A.
Authorship
All authors adhere to the following guidelines for authorship: ICMJE, Defining the Role of Authors and Contributors, Transparency in authors’ contributions and responsibilities to promote integrity in scientific publication, McNutt at all, PNAS February 27, 2018.
Author Contributions
All authors contributed to this original research article. All authors commented on the drafting of the manuscript. All authors read and approved the final manuscript.
Disclosures
Rosario Foti, Giorgia Giuffrida, Alice Ramondetta, Giorgio Amato, Elisa Visalli, Riccardo Foti, Francesco De Lucia, Ylenia Dal Bosco and Rocco De Pasquale have nothing to disclose.
Compliance with Ethics Guidelines
Our manuscript meets the ethical standards of the Declaration of Helsinki of 1964 and its later amendments. The authors certify that they have obtained all appropriate patient consent forms for the publication of case details and images. All subjects provided informed consent to participate in the study. The ethics committee of the University of Catania approved the study (170/11-02-2013).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Foti, R., Giuffrida, G., Ramondetta, A. et al. Multidisciplinary Rheuma–Derma Clinics: 5 Years of Experience at the San Marco Hospital in Catania. Dermatol Ther (Heidelb) 12, 2829–2837 (2022). https://doi.org/10.1007/s13555-022-00837-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00837-4