Abstract
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disorder involving decreased barrier function of the stratum corneum. This decrease, caused by a reduction in ceramide, the primary component of intercellular lipids in the stratum corneum, leads to a disturbance in the lamellar structure.
Methods
We developed a formulation (test cream) containing a steroid and synthetic pseudo-ceramide (SLE: N-(3-hexadecyloxy-2-hydroxypropyl)-N-2-hydroxyethyl hexadecanamide) that forms a lamellar structure on the skin after its application and drying. The formulation or control cream (a formulation containing a steroid but not pseudo-ceramide that does not form a lamellar structure) was applied twice daily for 2 weeks to the lesional area of 34 participants with mild to moderate AD symptoms.
Results
The test cream showed a periodic structure with an interface space of approximately 8.2 nm in transmission electron microscopy and small- and wide-angle X-ray scattering, similar to the lamellar structure in the human stratum corneum. In the double-blind test, the anti-inflammatory effects of the test cream (n = 17) were comparable to those of the control cream (n = 17). In the test cream group, a significant increase in the stratum corneum moisture content (p < 0.01) and significant decrease in transepidermal water loss (p < 0.05) were observed at weeks 1 and 2 after application compared with those before application. No such change was observed in the control group.
Conclusion
The results indicate that, even with a relatively short application period of 2 weeks, the test cream not only suppressed inflammation of the lesional area, but also improved the inherent barrier function of the stratum corneum, suggesting its potential as a treatment option for patients with AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Atopic dermatitis (AD) is an inflammatory skin disorder that presents with decreased barrier function in the stratum corneum. |
A decrease in the amount of intercellular lipids, the main component of which is ceramide, and changes in their structure contribute to the decreased barrier function of the stratum corneum in AD. |
We aimed to evaluate the efficacy of a cream containing a steroid and pseudo-ceramide that forms a lamellar structure in patients with AD. |
What was learned from the study? |
The test cream exhibited anti-inflammatory effects and improved the barrier function of the stratum corneum in the AD lesional area within a short period. |
The test cream containing pseudo-ceramide with a lamellar structure can be used as a treatment option for patients with AD. |
Introduction
Atopic dermatitis (AD) is an inflammatory skin disorder that presents with decreased barrier function in the stratum corneum, enabling irritants and allergens to easily infiltrate the skin and induce inflammatory and allergic responses. Therefore, skincare regimens that enhance barrier function in addition to treatment with drugs such as anti-inflammatory agents and anti-allergy medications are used [1, 2]. Typically, skincare consists of using moisturizing agents such as heparinoid, which reduce skin irritation and improve epidermal cell turnover, thereby improving the barrier function of the stratum corneum [3,4,5]. However, this effect is indirect and not immediate. Decreased barrier function of the stratum corneum is also observed in nonlesional areas; however, the decrease is considerably greater in lesional areas, where an inflammatory response is likely to recur with the infiltration of irritants, even with drug-induced suppression of inflammation [6]. In addition, Staphylococcus aureus releases aggravating factors in AD [7]. Therefore, it is desirable to immediately enhance the inherent barrier function of the skin at lesional sites, which exhibit poor stratum corneum functions (i.e., moisture and barrier) and are prone to inflammation recurrence.
The barrier function of the stratum corneum is attributed to the presence of intercellular lipids [8] derived from extracellular release of lamellar granules from epidermal cells [9]. In AD, the size of lamellar granules and number of released lamellar granules decrease [10]. In addition, the level of ceramides, the primary components of these intercellular lipids, is diminished in AD. Furthermore, the composition of fatty acids and ceramides is altered [11, 12], resulting in an abnormal intercellular lipid structure in the stratum corneum of individuals with AD [13, 14]. A decrease in the amount of intercellular lipids and changes in their structure are thought to contribute to the decreased barrier function of the stratum corneum in AD.
AD symptoms improve following supplementation of synthesized pseudo-ceramide (SLE: N-(3-hexadecyloxy-2-hydroxypropyl)-N-2-hydroxyethyl hexadecanamide (Fig. 1)) or ceramides [15,16,17,18,19,20,21,22,23,24,25]. We hypothesized that improving the barrier function of the stratum corneum by supplementation of SLE and delivering agents that form a lamellar structure similar to that of the intercellular lipids would improve AD. An SLE-containing steroid formulation that forms a lamellar structure on the skin surface after application and drying may be useful for suppressing inflammation in the lesional area and improving the barrier function of the stratum corneum in patients with AD. Therefore, we developed a steroid formulation and evaluated its usefulness in ameliorating the barrier function of the stratum corneum in the lesional areas of patients with AD.
Methods
Test Cream
The test cream was an oil-in-water cream formulation containing 0.15% prednisolone valerate acetate (PVA) and 3% synthetic pseudo-ceramide. The other components were cetyl alcohol, stearyl alcohol, polyoxyethylene sorbitan monostearate, sorbitan monostearate, glycerin, and water. The control cream was an oil-in-water-type commercial steroid cream containing 0.15% PVA, based on Vaseline, with cetyl alcohol, stearyl alcohol, polyoxyethylene sorbitan monostearate, and sorbitan monostearate.
Structural Analysis of the Formulation Film
Specimen Preparation for Transmission Electron Microscopy
The structures of the dried films of the test and control creams were observed as follows. Briefly, each cream was applied with a coater (thickness of 120 μm) on a polyethylene terephthalate overhead projector film. After 3 h of drying, 5-mm squares were cut out, stained, and fixed with osmium tetroxide for 24 h at room temperature (23 °C). A Leica UC-6 cryo-ultramicrotome (Leica Microsystems, Wetzlar, Germany) was used to obtain ultrathin sections of 50–100-nm thickness at −80 °C. The sections were collected in droplets of frozen 2.3-M sucrose, placed on a grid (200 mesh with support film, without carbon reinforcement), washed, and dried. H-7650 (Hitachi, Tokyo, Japan) was used to perform measurements at an acceleration voltage of 80–100 kV. A charge-coupled device (1024 × 1024 pixels) was used for detection.
Small- and Wide-Angle X-Ray Scattering
The dried films of the test and control creams were evaluated using a small- and wide-angle X-ray scattering apparatus (SAXSess mc2; Anton Paar GmbH, Graz, Austria). For sampling of the dried film, the substance was applied on a polyethylene terephthalate resin overhead projector film with a coater (120 μm thickness), dried for 3 h, scraped with a spatula, and placed into cells. Paste cells specific for SAXSess mc2 were used as sample cells. X-Ray scatter detection was performed using an imaging plate for SAXSess mc2. The measurement conditions were as follows: X-ray wavelength, 0.1542 nm; sample–detector distance, 259.2 mm; and measurement temperature, 25 °C. The exposure time was set at 5–60 min on the basis of the balance between the irradiation limit of the Imagine Plate and detection sensitivity (signal-to-noise ratio). After exposure to the imaging plate, on the basis of the acquired two-dimensional scattering images, a one-dimensional scattering profile was created using the accessory software provided with SAXSess mc2 (SAXSquant 2D; SAXSquant, Anton Paar GmbH). The period d [nm] of the lamellar structure observed on X-ray scattering was determined from the peak position as follows: q = 2π/d [nm−1], where q is the scattering vector.
Efficacy Test
Subjects
A parallel, randomized, double-blind, dermatologist-controlled baseline comparison study was performed from May to November 2016 in Hokkaido, Japan (UMIN registration no. 000022212). The study adhered to the tenets of the Declaration of Helsinki, and all clinical studies were reviewed and approved by the Review Board of Kao Corporation (Tokyo, Japan) and Medical Corporation Kojinkai Sapporo Skin Clinic (Sapporo, Japan). Written informed consent was obtained from each subject prior to the study.
Subjects with mild to moderate skin symptoms of AD on the inner forearm were prescreened from candidates by visual observation and medical interviews conducted by dermatologists. Subjects who received medical treatment on their face were excluded. Among the 59 Japanese patients who were prescreened, 36 subjects aged 16–49 years (mean, 28.9 ± 0.9 years) were enrolled in this study.
Among the patients with AD (age range, 16–49 years) presenting with mild to moderate grade at the test site (inside of the left forearm), who fully understood the significance, content, and purpose of the study and wished to participate in this study, 36 were selected as eligible candidates by the investigator and study director.
Evaluation Flow
After screening, the 36 selected participants were divided into the test formulation application group (n = 18) and control cream application group (n = 18) on the basis of age, sex, and dermatologist evaluation indices.
After a 1-week washout period (−1 W), the application of the test formulation was started, and the participant was interviewed by a dermatologist. The test site was visually evaluated, measured, and photographed at the start of test formulation application (W0), 1 week (W1), and 2 weeks (W2). Severity assessment using photographs was conducted after the application period (Fig. 2).
During the washout period, the subjects were requested to abstain from applying any formulations such as moisturizing agents or medications to the test site and to refrain from taking other medications for AD. However, if the skin condition worsened markedly during this period, the subject was excluded from the study, and the patient received appropriate treatment from a dermatologist.
Application of Test or Control Cream
Test cream (approximately 0.5 g) or control cream was applied to the forearm twice daily, once in the morning and once at night, for 2 weeks. The participants were allowed to apply the drug to non-test sites. When abnormalities were observed in the application site during the study, the dermatologist provided appropriate treatment, and drug use was discontinued as necessary. Application of other moisturizing agents or topical agents to the test site during the usage period was prohibited. Switching of cleansing agents was not allowed. The application of skincare products at non-test sites was allowed.
Evaluation and Measurement
After washing the test site with water and thoroughly wiping off the moisture, the subjects were acclimatized at 20 ± 2 °C and 45.0 ± 5.0% humidity for at least 20 min before evaluation and measurement. The same location was measured every time by making a transparent sheet showing the measurement site for each subject. The sheet was applied on their arms, and the measurement site was marked before acclimatization.
Interview, Examination, and Severity Assessment
Interviews and examinations were performed at W0, W1, and W2 visits. The test sites were photographed with a Canon EOS Kiss X5 camera (lens: EF-S60mm f/2.8 Macro USM, Canon, Tokyo, Japan). Severity assessment of the test sites was performed using the photographs taken at W0, W1, and W2 after W2 examination. A dermatologist assigned severity scores of 1–4 (1, mild; 2, moderate; 3, severe; 4, very severe) based on the severity classification (simple method) of AD, which is comprehensively determined by the degree and areas of eruption considering these eight factors: erythema, papules, erosion, crusts, excoriation, lichenification, pruriginous nodules, and depilation. It was developed by the advisory committee of the AD severity classification of the Japanese Dermatological Association [26].
Skin Property Measurement
The moisture content in the stratum corneum at each test site was measured using SKICON-200EX (IBS, Hamamatsu, Japan). The test site was measured at five points, and the mean from the middle three values (after excluding the highest and lowest values) was defined as the measurement value. Transepidermal water loss (TEWL) was measured at one point in the test site on the left arm using Tewameter TM300 (Courage + Khazaka electronic GmbH, Köln, Germany).
Statistics
Significance analysis was performed after determining the target participants for effectiveness evaluation based on a usage rate (%) of at least 80% of the test formulation and compliance status. For significance testing, the results of photographic severity assessment were analyzed using Mann–Whitney U test between groups and Scheffé’s multiple comparison test within groups. Skin property measurement values were calculated as the changes from the start date (W0). The results were analyzed using Student’s t-test between groups and the Holm–Bonferroni method for within-group comparisons. Statistical significance was set at a 95% confidence interval (P < 0.05). Statistical analyses were performed using SPSS Statistics software (version 19; SPSS, Inc., Chicago, IL, USA) and Ekuseru-Toukei 2012 (Social Survey Research Information Co., Ltd, Tokyo, Japan).
Results
Structural Analysis of Film Formulation
The lamellar structure of the test cream was confirmed using transmission electron microscopy. A layered structure with intervals of less than 10 nm was observed (Fig. 3). However, no such lamellar structure was observed for the control cream (data not shown). The X-ray analysis was performed to confirm the detailed lamellar structure. The X-ray one-dimensional scattering profile of the dried test cream on the film showed a peak at q = 0.765 nm−1 [Fig. 4A (a)]. The periodicity phase of the test cream was approximately 8.2 nm, and a lamellar structure was formed after application and drying. No clear peak was observed within the q = 0.05–1 nm−1 range for the control cream (Fig. 4B), whereas a clear peak was observed at q = 15.4 nm−1 for the test cream [Fig. 4A (b)], although multiple peaks appeared in this position for the control cream [Fig. 4B (c)]. This result indicates that the test cream had an α-gel structure, and some intercellular lipids in the human stratum corneum have been shown to have the same structure. These results demonstrate that a lamellar structure was formed after applying the test cream.
Structure of the test and control cream analyzed using X-ray scattering analysis. A The test cream (red line) showed a peak at q = 0.765 nm−1 and a lamellar structure with a periodicity phase of approximately 8.2 nm (a). A clear peak was also observed at q = 15.4 nm−1 in the test cream (b). B The control cream (blue line) showed no clear peak within the q = 0.05–1 nm−1 range. Multiple peaks from crystal structures appeared around q = 15.4 nm−1 in the control cream (c)
Efficacy Test
During the 1-week washout period before application, none of the participants exhibited progression in the condition that required treatment, and all 36 participants applied the formulation for 2 weeks. However, a subject in the control cream group could not appear at W2 visit and a subject in the test cream group did not apply the cream twice per day. Therefore, these subjects were excluded, and the efficacy in and skin properties of the remaining control cream group (n = 17) and test cream group (n = 17) subjects were evaluated.
Safety and Efficacy
Interview and examination by a dermatologist did not reveal drug-related adverse effects of either the test cream or control cream, suggesting comparable effectiveness and safety between the creams.
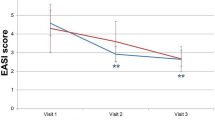
The photographic severity assessment showed that 2 weeks of application significantly improved symptoms compared with those before application in both test and control groups (p < 0.01 for both). However, no significant difference in the degree of improvement was evident between the groups (Fig. 5).
Measurement of Skin Properties
The moisture content in the stratum corneum in the test and control cream groups is shown in Fig. 6A. In the test cream group, the changes in the skin hydration value compared with that at W0 were 48.0 ± 13.0 at W1 and 44.7 ± 11.2 at W2, indicating significant increases [mean ± standard error (SE), p < 0.01 at both W1 and W2]. In contrast, the values of the control cream group were −3.4 ± 3.6 at W1 and 3.8 ± 5.5 at W2, indicating no significant changes. The changes in skin hydration values at weeks 1 and 2 in the test cream group were significantly higher than those in the control cream group (p < 0.01).
Changes in skin hydration and transepidermal water loss. A No significant changes in the control group (n = 17). Significant increase in the test cream group (n = 17) (mean ± SE, **p < 0.01, Holm–Bonferroni method, versus week 0). The changes in the skin hydration value at weeks 1 and 2 in the test cream group were significantly higher than those in the control cream group (††p < 0.01, Student’s t-test). B Transepidermal water loss significantly decreased in the test cream group following application (mean ± SE, *p < 0.05, Holm–Bonferroni method, versus week 0). The changes in transepidermal water loss in the test cream group at weeks 1 and 2 were significantly lower than those in the control cream group (†p < 0.05, Student’s t-test)
The TEWL values of the test and control cream groups are shown in Fig. 6B. The changes in TEWL from that at W0 in the test cream group were −2.1 ± 0.9 and −2.2 ± 1.0 g/hm2 at W1 and W2, respectively, showing a significant decrease compared with that before application (mean ± SE, p < 0.05 at both W1 and W2). In contrast, the values of the control cream group were 0.1 ± 0.4 and 0.4 ± 0.5 g/hm2 at W1 and W2, respectively, indicating no significant changes. The changes in TEWL in the test cream group at weeks 1 and 2 were significantly lower than those in the control group (p < 0.05).
Discussion
On the basis of the efficacy test, the formulation that formed a lamellar structure on the skin after drying displayed effects comparable to those obtained with the formulation that did not form such a structure, improving AD eczema (Fig. 5). This was likely because we included patients who presented with relatively mild AD symptoms (mild to moderate grade). Therefore, although PVA (a relatively weak steroid) was used, both treatments resulted in apparent improvements [27]. We also evaluated permeability in an infinite capacity system using porcine skin and verified that the test and control creams showed permeability comparable to that of PVA at 24 h after application (data not shown). These results indicate that the test cream was as effective and safe as the control cream.
The severity assessment revealed comparable improvements; however, skin hydration significantly increased and the TEWL significantly decreased with the test cream compared with the control cream (Fig. 6). These results indicate that the test formulation possessing a lamellar structure improves the stratum corneum function.
In general, to stably emulsify a steroid with high crystallinity, dissolution in polar oils is essential. However, polar oils can easily destroy the lamellar structure of the formulation. Therefore, preparing a steroid formulation that maintains the lamellar structure after drying is extremely challenging. However, we identified a stable emulsification region and developed this formulation by combining a steroid and SLE with multiple higher alcohols and surfactants with different critical packing parameters [28, 29].
In this study, we confirmed that the periodicity phase of the lamellar layer formed on the skin after applying and drying the test cream was approximately 8.2 nm (Figs. 3, 4). The periodic structure of lamella was observed in the human stratum corneum, and the structure is important to maintaining barrier and moisturizing function [30,31,32]. It was confirmed that this formulation also had a lamella structure, although the distance of the lamellar phases was slightly different. However, by having a lamella structure, the test cream should contribute to improve barrier and moisturizing function.
After application and drying the test cream, a lamellar structure coated the skin. This not only led to anti-inflammatory effects, but also increased the barrier and moisturizing functions, normalizing epidermal turnover more rapidly. This may explain the improvements in stratum corneum function. When covering the skin, a complete seal would result in maceration, the tissue destruction from long-term exposure to high humidity, of the healthy surrounding tissue, delaying barrier healing. Therefore, an appropriate moisture permeability is necessary [33,34,35,36]. The lamellar structure formed by the test cream after application and drying, unlike that observed with plastic wrap that completely seals objects, exhibits adequate moisture permeability, thus functioning as a physical protective film on the skin. Following disruption of the skin barrier with acetone ether, a formulation with a lamellar structure promotes barrier recovery [28]. Therefore, the test cream, even after a relatively short application period of 2 weeks, promoted and normalized ceramide production and turnover within the skin at the lesional area by ensuring appropriate occlusion and improved the barrier function of the stratum corneum in participants.
The skin of patients with AD shows a markedly decreased stratum corneum barrier function in both lesional and nonlesional areas compared with the skin of healthy individuals [6]. Thus, even after the management of dermatitis with steroid treatment, the abnormal barrier function in the nonlesional area does not normalize. The test cream improved the inherent barrier function of the skin through its anti-inflammatory effects as a drug, as well as by its high-moisturizing function as a skincare product. Proactive therapy for AD requires thorough suppression of inflammation by applying steroids to the non-lesional area and improving the function of the stratum corneum [37]. For this reason, the cream contained anti-inflammation agents and pseudo-ceramide with a lamellar structure may be useful for proactive therapy, but since the side effects of long-term use of steroids are also of concern, it is necessary to consider the period of use when using steroids as anti-inflammatory agents.
In addition, it is possible to reduce the steroid dose or use a weaker class (potency) of steroids. The compliance rate with topical agents is lower in patients with AD than in patients with other dermatitis conditions [38, 39]. The test cream does not require the use of other topical agents or skincare agents, even during the AD exacerbation period, and therefore may improve drug adherence. Maintenance of the remission period after discontinuation of the drug and the possibility of lowering the steroid class should be further studied.
Conclusions
The cream containing a steroid and pseudo-ceramide that forms a lamellar structure not only showed anti-inflammatory effects but also greatly improved barrier function in patients with mild to moderate AD within a short period. This cream should be useful in AD, which is complicated by inflammation and reduced barrier function.
References
Saeki H, Furue M, Furukawa F, Hide M, Ohtsuki M, Katayama I, et al. Guidelines for management of atopic dermatitis. J Dermatol. 2009;36:563–77. https://doi.org/10.1111/j.1346-8138.2009.00706.x.
Katayama I, Kohno Y, Akiyama K, Ikezawa Z, Kondo N, Tamaki K, et al. Japanese guideline for atopic dermatitis. Allergol Int. 2011;60:205–20. https://doi.org/10.2332/allergolint.11-rai-0333.
Lodén M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771–88. https://doi.org/10.2165/00128071-200304110-00005.
Tagami H, Ohi M, Iwatsuki K, Kanamaru Y, Yamada M, Ichijo B. Evaluation of the skin surface hydration in vivo by electrical measurement. J Invest Dermatol. 1980;75:500–7. https://doi.org/10.1111/1523-1747.ep12524316.
Kikuchi K, Tagami H. Japanese Cosmetic Scientist Task Force for Skin Care of Atopic Dermatitis. Noninvasive biophysical assessments of the efficacy of a moisturizing cosmetic cream base for patients with atopic dermatitis during different seasons. Br J Dermatol. 2008;158:969–78. https://doi.org/10.1111/j.1365-2133.2008.08478.x.
Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–6. https://doi.org/10.1111/1523-1747.ep12470233.
Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity. 2015;42:756–66. https://doi.org/10.1016/j.immuni.2015.03.014.
Van Smeden J, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta. 2014;1841:295–313. https://doi.org/10.1016/j.bbalip.2013.11.006.
Elias PM, Goerke J, Friend DS. Mammalian epidermal barrier layer lipids: composition and influence on structure. J Invest Dermatol. 1977;69:535–46. https://doi.org/10.1111/1523-1747.ep12687968.
Fartasch M, Bassukas ID, Diepgen TL. Disturbed extruding mechanism of lamellar bodies in dry non-eczematous skin of atopics. Br J Dermatol. 1992;127:221–7. https://doi.org/10.1111/j.1365-2133.1992.tb00118.x.
Ishikawa J, Narita H, Kondo N, Hotta M, Takagi Y, Masukawa Y, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511–4. https://doi.org/10.1038/jid.2010.161.
van Smeden J, Janssens M, Kaye EC, Caspers PJ, Lavrijsen AP, Vreeken RJ, et al. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp Dermatol. 2014;23:45–52. https://doi.org/10.1111/exd.12293.
Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136–8. https://doi.org/10.1038/jid.2011.175.
Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53:2755–66. https://doi.org/10.1194/jlr.P030338.
Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219–23. https://doi.org/10.1007/BF01106105.
Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27–30. https://doi.org/10.1080/00015559850135788.
Imokawa G, Ishida K. Role of ceramide in the barrier function of the stratum corneum, implications for the pathogenesis of atopic dermatitis. J Clin Exp Dermatol Res. 2014;5:1000206.
Berardesca E, Barbareschi M, Veraldi S, Pimpinelli N. Evaluation of efficacy of a skin lipid mixture in patients with irritant contact dermatitis, allergic contact dermatitis or atopic dermatitis: a multicenter study. Contact Dermatitis. 2001;45:280–5. https://doi.org/10.1034/j.1600-0536.2001.450505.x.
Chamlin SL, Frieden IJ, Fowler A, Williams M, Kao J, Sheu M, et al. Ceramide-dominant, barrier-repair lipids improve childhood atopic dermatitis. Arch Dermatol. 2001;137:1110–2.
Seghers AC, Cai SC, Ho MS, Giam YC, Tan L, Grönhagen CM, et al. Evaluation of a pseudoceramide moisturizer in patients with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2014;4:83–92. https://doi.org/10.1007/s13555-014-0048-z.
Simpson E, Böhling A, Bielfeldt S, Bosc C, Kerrouche N. Improvement of skin barrier function in atopic dermatitis patients with a new moisturizer containing a ceramide precursor. J Dermatolog Treat. 2013;24:122–5. https://doi.org/10.3109/09546634.2012.713461.
Imokawa G, Akasaki S, Kawamata A, Yano S, Takaishi N. Water retaining function in the stratum corneum and its recovery properties by synthetic pseudoceramides. J Soc Cosmet Chem. 1989;40:273–85.
Hata M, Tokura Y, Takigawa M, Tamura Y, Imokawa G. Efficacy of using pseudoceramide-containing cream for the treatment of atopic dry skin in comparison with urea cream. Nishinihon J Dermatol. 2002;64:606–11. https://doi.org/10.2336/nishinihonhifu.64.606.
Matsuki H, Kiyokane K, Matsuki T, Sato S, Imokawa G. Recharacterization of the nonlesional dry skin in atopic dermatitis through disrupted barrier function. Exog Dermatol. 2004;3:282–92. https://doi.org/10.1159/000091909.
Matsuki H, Kiyokane K, Matsuki T, Sato S, Imokawa G. Reevaluation of the importance of barrier dysfunction in the nonlesional dry skin of atopic dermatitis patients through the use of two barrier creams. Exog Dermatol. 2004;3:293–302. https://doi.org/10.1159/000091910.
Saeki H, Furue M, Furukawa F, et al. Guidelines for management of atopic dermatitis. J Dermatol. 2009;36:563–77. https://doi.org/10.1111/j.1346-8138.2009.00706.x.
Eichenfield LF, Tom WL, Berger TG, et al. Guideline of care for the management of atopic dermatitis. Section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116–32.
Orita M, Uchiyama M, Hanamoto T, Yamashita O, Naitou S, Takeuchi K, et al. Formation of pseudo-intercellular lipids membrane on the skin surface by the alpha-gel holding a large amount of water. J Soc Cosmet Chem Jpn. 2012;46:25–32. https://doi.org/10.5107/sccj.46.25.
Yamaguchi M, Takahashi M, Harusawa F, Fukushima S. Mechanism of stabillization by cetyl alcohol for oil in water cream. J Soc Cosmet Chem Jpn. 1978;12:16–22. https://doi.org/10.5107/sccj1976.12.2_16.
Pilgram GS, Bouwstra JA. Stratum corneum lipid organization in vitro and in vivo as assessed by diffraction methods. In: Forslind B, Lindberg M, editors. Skin, hair, and nails: structure and function. New York: Marcel Dekker; 2004.
Hatta I, Nakanishi K, Ohta N. Structure and phase transitions of intercellular lipid assembly in stratum corneum. Netsu Sokutei. 2004;34:159–66.
Ohta N, Ban S, Tanaka H, Nakata S, Hatta I. Swelling of intercellular lipid lamellar structure with short repeat distance in hairless mouse stratum corneum as studied by X-ray diffraction. Chem Phys Lipids. 2003;123:1–8. https://doi.org/10.1016/s0009-3084(02)00126-3.
Junker JP, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv Wound Care. 2013;2:348–56. https://doi.org/10.1089/wound.2012.0412.
Zhai H, Maibach HI. Effect of occlusion and semi-occlusion on experimental skin wound healing: a reevaluation. Wounds. 2007;19:270–6.
Zehrer CL, Holm D, Solfest SE, Walters SA. A comparison of the in vitro moisture vapour transmission rate and in vivo fluid-handling capacity of six adhesive foam dressings to a newly reformulated adhesive foam dressing. Int Wound J. 2014;11:681–90. https://doi.org/10.1111/iwj.12030.
Feingold KR, Denda M. Regulation of permeability barrier homeostasis. Clin Dermatol. 2012;30:263–8. https://doi.org/10.1016/j.clindermatol.2011.08.008.
Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011;164:415–28. https://doi.org/10.1111/j.1365-2133.2010.10030.x.
Murota H, Takeuchi S, Sugaya M, Tanioka M, Onozuka D, Hagihara A, et al. Characterization of socioeconomic status of Japanese patients with atopic dermatitis showing poor medical adherence and reasons for drug discontinuation. J Dermatol Sci. 2015;79:279–87. https://doi.org/10.1016/j.jdermsci.2015.05.010.
Furue M, Onozuka D, Takeuchi S, Murota H, Sugaya M, Masuda K, et al. Poor adherence to oral and topical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272–5. https://doi.org/10.1111/bjd.13377.
Acknowledgements
We appreciate the staff at Dermalabo Co., Ltd. for their cooperation during the study.
Funding
This study was fully funded by Kao Corporation, Japan. The journal’s Rapid Service Fee was also sponsored by Kao Corporation, Japan.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Keita Okoshi, Yoshinori Kinugasa, Takuji Kume, Tsuyoshi Seki, and Azumi Nagasawa. The first draft of the manuscript was written by Yoshinori Kinugasa and Shotaro Ito; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Keita Okoshi, Yoshinori Kinugasa, Shotaro Ito, Takuji Kume, Tsuyoshi Seki, Takahiro Nishizaka, Joji Okada, Hiromitsu Kawada, Azumi Nagasawa, Makoto Iijima, Masatoshi Abe, and Osamu Nemoto have nothing to disclose
Compliance with Ethics Guidelines
The human study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The study was reviewed and approved by the Review Board of Kao Corporation (Tokyo, Japan) and Medical Corporation Kojinkai Sapporo Skin Clinic (Sapporo, Japan). All participants provided informed consent prior to inclusion in the study.
Data Availability
The data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Okoshi, K., Kinugasa, Y., Ito, S. et al. Efficacy of Pseudo-Ceramide-Containing Steroid Lamellar Cream in Patients with Mild to Moderate Atopic Dermatitis: A Randomized, Double-Blind Study. Dermatol Ther (Heidelb) 12, 1823–1834 (2022). https://doi.org/10.1007/s13555-022-00766-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00766-2