Abstract
Introduction
Although alopecia areata (AA) profoundly impacts patients’ physical appearance, emotional state, and daily activities, no treatment approved for AA currently exists. Patient-reported outcome (PRO) instruments currently used to capture patients’ AA experiences do not meet the requirements to support claims of treatment benefit as described in the US Food and Drug Administration’s 2009 PRO guidance. Our objective was to explore the consequences and priority treatment outcomes among individuals with AA and develop a PRO measure consistent with regulatory requirements that assesses these priorities and represents clinical benefit from the AA patient perspective.
Methods
Targeted literature and instrument reviews informed an initial concept set. Concept elicitation interviews with 20 adults with AA confirmed the relevance and importance of the initial concepts, identified additional relevant concepts, and informed an AA consequence model. Thematic analysis yielded a draft item pool, which was evaluated through two iterative rounds of cognitive debriefing interviews with 16 patients with AA (9 adults; 7 adolescents).
Results
Hair loss was the primary consequence of importance to patients with AA. Patients emphasized the need to differentiate hair loss by location: scalp, eyebrows, eyelashes, and body. Consequences of AA include difficulty conducting daily activities, particularly outdoor activities and exercise, and emotional impacts such as sadness, frustration, and negative self-image. Following cognitive debriefing interviews, 11 items were included to form the Alopecia Areata Patient Priority Outcome (AAPPO), assessing AA-related symptoms and impacts over the past week.
Conclusions
The AAPPO is a novel, content-valid PRO that captures the consequences of AA of the highest priority to patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with alopecia areata (AA) experience profound impacts on their physical appearance, emotional state, and daily activities and face unmet treatment needs. None of the available patient-reported outcome (PRO) measures currently used to evaluate patients’ experiences with AA were developed in line with regulatory requirements. |
Based on qualitative research with adults and adolescents with AA and a review of the published evidence, a novel AA-specific PRO measure was developed to capture the consequences and priority treatment outcomes of individuals with AA. |
Cognitive debriefing interviews with patients confirmed the content validity of the measure, which was developed in a manner consistent with regulatory guidance. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14054123.
Introduction
Alopecia areata (AA), a T-cell-mediated disease characterized by nonscarring hair loss, is one of the most common autoimmune diseases for both men and women [1]. People living with AA are at a higher risk than the general population of developing depression, anxiety, and social phobia [2]. Living with AA has also been associated with much higher levels of body dissatisfaction and concern with general appearance due to the associated hair loss [2].
Treatment for AA typically involves off-label use of topical, intralesional, and systemic corticosteroids as well as non-steroidal treatments such as topical immunotherapy, topical minoxidil, topical irritants such as anthralin, and systemic immunosuppressants such as cyclosporine or methotrexate [3]. This strategy yields limited success, as most patients with AA relapse after therapy, and long-term use of these treatments is associated with concerns regarding tolerability and safety [4]. The symptomatic and emotional consequences of AA, coupled with a lack of highly effective treatment options, represent a significant unmet medical need [1].
In the age of patient-focused drug development (PFDD), instruments that assess benefit in clinical trials of investigational AA therapies must address outcomes and consequences of greatest priority to patients and adhere to regulatory requirements for development [5]. The objectives of this study were to (i) explore the consequences and priority treatment outcomes of adults and adolescents with AA and, if necessary, (ii) develop a patient-reported outcome (PRO) measure consistent with current regulatory expectations and representing clinical benefit from the AA patient perspective [6].
Methods
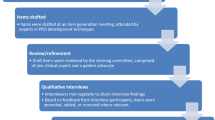
This research was conducted in two stages: concept exploration and content confirmation. Following regulatory guidance on development of a PRO intended for use in a clinical trial setting to assess clinical benefit from the patient perspective (i.e., its context of use), concept exploration included reviews of existing data sources, followed by concept elicitation (CE) interviews with AA patients to identify patient priority concepts. Results from this first stage informed generation of a disease consequence model as well as a set of draft items. These items were then evaluated during content confirmation, which included cognitive debriefing (CD) interviews to ensure that the items assess patient priority outcomes in a manner reflective of how they are experienced. The qualitative research study materials were reviewed and approved by the RTI Institutional Review Board (Approval No. 14241). The study complied with the Declaration of Helsinki, and all interview participants provided informed consent.
Content Exploration
Concepts that should be considered for inclusion in an AA-specific PRO were explored through targeted reviews of the literature and the ePROVIDETM clinical outcomes assessment (COA) repository, as well as the 11 September 2017 FDA Patient-Focused Drug Development (PFDD) public meeting on AA [7, 8].
Literature, Instrument, and Patient-Focused Drug Development Meeting Materials Review
A literature search was conducted within PubMed to identify articles focusing on concepts and experiences associated with living with AA. The search (Table 1) targeted articles containing information on concepts of importance from the patient perspective. Articles were included if they contained the relevant patient population and data related to patient-reported symptom or impact concepts.
In addition, a desktop search of the ePROVIDETM database of COA instruments was conducted to identify AA-specific PRO instruments to inform the list of relevant patient concepts further(ePROVIDE, 2018) [7]. Study staff also attended the 11 September 2017 FDA PFDD public meeting on AA and reviewed the meeting report [8] and transcript [9] to identify any additional priority concepts to persons with AA discussed during this meeting not represented in the literature and instrument reviews.
AA Consequence Model
An AA consequence model was drafted based on the literature and instrument reviews and the FDA PFDD meeting materials and refined based on data collected during the CE interviews. The model provided a framework for presenting the consequences of AA that represent priorities for treatment from the AA patient perspective.
CE Interviews
Patient interviews were conducted to confirm the AA consequence model and identify any missing concepts. Patient databases from two research organizations (L&E Research and Shifrin Hayworth) were utilized to identify participants for in-person CE interviews at three US locations (Charlotte, North Carolina; Tampa, Florida; and Southfield, Michigan). Medical recruiters employed a structured screening questionnaire to determine study eligibility. Eligible individuals were aged 12 years or older, were able to speak and read English, had self-reported a clinician diagnosis of AA, ≥ 50% hair loss, and had a sustained period of hair loss for ≥ 2 years. Although adolescents (aged 12–17 years) were eligible for the CE interviews, none could be recruited; however, adolescents did participate in a CE task during subsequent CD interviews. Individuals with other forms of alopecia (e.g., traction or scarring alopecia) or active forms of other inflammatory skin diseases (e.g., psoriasis, seborrheic dermatitis, lupus) were excluded.
The interviews were led by two experienced qualitative researchers (SM and NH, one of whom led the interviews and the other took notes) and followed a semi-structured interview guide. The interviews began with open-ended questions about participants’ experiences with AA (e.g., the initial signs, symptoms, and how this onset of disease affected their daily life). Participants were then asked questions to elicit additional concepts that were found in other content exploration sources but not spontaneously reported during the open-ended portion of the interview. Each interview lasted approximately 60 min and was audio recorded and transcribed. Additional CE interviews were conducted until saturation was reached (i.e., no new concepts emerged).
Content Confirmation
Based on the CE findings from the content exploration stage of work and a review of existing instruments, de novo instrument generation work was deemed necessary. The consequences of AA that were most frequently endorsed by participants and described as most impactful were selected for inclusion in a draft PRO measure. The draft set of items was tested during CD interviews with adult and adolescent participants to confirm that they adequately captured the concepts of priority to AA patients.
CD Interviews
Participants in the CD interviews were identified and recruited by a board-certified practicing dermatologist based in Fairfield, Connecticut. The CD interviews were conducted via telephone so that the interviews could be scheduled at the participants’ convenience and to expedite collection of content confirmation evidence so that the measure could be finalized for inclusion in a planned clinical trial. Enrolled participants resided in Connecticut (n = 8), New York (n = 5), Ohio (n = 1), Massachusetts (n = 1), and Pennsylvania (n = 1). Inclusion and exclusion criteria were identical to those for recruiting the CE sample, with the exception that all CD participants had a dermatologist-confirmed diagnosis of AA and had experienced a sustained period of hair loss for ≥ 6 months.
The same interviewers (SM and NH) conducted the CD interviews following a semi-structured interview guide. The adult interviews began with a brief exploration of the participant’s experiences with AA and the consequences considered to be of highest priority. As the initial CE set was composed solely of adults, the adolescent participants (i.e., those aged 12–17 years) in this set of interviews first engaged in a detailed CE discussion. All CD participants were debriefed on the draft items using a “think aloud” format that allowed interviewers to gather information about participants’ interpretations of each item [10]. Specifically, after providing their feedback on the title and instructions, participants read each item while describing their thought processes out loud (i.e., understanding of the question and reasons for selecting a specific response option). Interviewers also asked probing questions about participants’ interpretation of the questions, recall period, and response options to identify any need for modifications to improve comprehension and ease of response. Upon completion of the measure, participants were asked whether any item(s) could be omitted and whether any important concepts were missing from the draft item set. The wording and response options of the draft items were refined after the first set of CD interviews. Adult interviews lasted approximately 60 min and adolescent interviews lasted approximately 90 min due to the inclusion of the CE portion; all interviews were audio recorded and transcribed.
Data Analysis
Thematic analysis of the qualitative data was conducted in a standard, systematic manner. For CE interviews, the symptom and impact consequences described during each interview were tabulated, with the aim of documenting concept saturation (i.e., the point at which no new priority consequences emerged) [6, 10]. In addition, participant quotations characterizing the symptom and impact consequences to be included in the item set were documented.
After each round of CD interviews, the interviewers analyzed their field notes to identify any potential problems within the questionnaire. Specifically, the results of the first round of CD interviews were reviewed to summarize and identify patterns in the way participants interpreted and responded to each item and determine how well the items captured consequences of priority to the participants. The authors then implemented revisions to the draft questionnaire based on the interview results. The revised questionnaire was evaluated in the second round of interviews. An item-tracking matrix was also produced for each round of interviews to document revisions made to the items and the rationale for the revisions.
Results
Content Exploration
Literature, Instrument, and Patient-Focused Drug Development Meeting Materials Review.
Of 108 abstracts found in PubMed, 43 were deemed potentially relevant and reviewed in full-text form. Ultimately, 25 articles were included, most of which were observational studies, while 4 included a qualitative component. In addition to the hallmark symptom of hair loss, a broad range of potential impacts of AA were reported, including emotional symptoms (e.g., bullying, perceived stigma, and functional limitations such as difficulty engaging in daily activities).
Across all of the observational and mixed methods studies reviewed, the most commonly utilized COA measures were the Dermatology Life Quality Index (DLQI) [11] (n = 7), Skindex (n = 6; includes the 16-item [n = 3], 17-item [n = 1], and 29-item [n = 2] versions) [12,13,14], and the 36-item Short Form Health Survey (SF-36) [15, 16] (n = 5). Importantly, none of these measures were developed to capture the AA patient experience. The first two were designed to assess general dermatologic skin conditions and thus have item wording that is not appropriate for the AA patient (e.g., items ask about “skin” rather than “hair”). The SF-36 is a generic measure of health status and does not fully capture the range of concepts that are likely of priority to the AA patient. Full-text article review and a desktop search of ePROVIDETM revealed three additional measures that were developed specifically for the AA population: the Alopecia Areata Symptom Impact Scale (AASIS) [17], the Alopecia Areata Quality of Life (AAQ) [18], and the Alopecia Areata Quality of Life Index (AA-QLI) [15, 16, 19].
Findings from the PFDD meeting revealed the most significant AA symptoms to be hair loss on the scalp, hair loss on other areas of the body, and sensitivity to sun [7]. Impacts of AA included activity limitations and emotional symptoms such as depression, anxiety, and perceived stigma [7].
CE Interviews
A total of 20 patients participated in the CE interviews. Average age of these participants was 52 years, and participants had been experiencing AA for an average of nearly 20 years. Most CE interview participants were female (n = 16; 80%), and 60% were African American (n = 12). Participants ranged in disease severity (Table 2).
During the CE portion of the second set of interviews, adolescents provided feedback similar to that provided by adult CE participants. Descriptions of the onset of AA included an initial experience of diffuse hair thinning or discrete patches of complete hair loss. Participants detailed the marked emotional impact of both the diagnostic process as well as experiences with treatments (both prescribed and nonprescribed) and noted that treatment had little to no positive effect on their hair loss. When exploring beyond the initial scalp hair loss, participants spontaneously reported very few additional symptoms or signs (Table 3). Loss of eyebrow and eyelash hair were the most common spontaneously reported signs (n = 6 for each). When discussing hair loss, participants emphasized the need to differentiate each location separately, including scalp hair, eyebrows, eyelashes, and body hair. Other scalp hair signs—such as white hair and patchy hair regrowth—were less frequently reported and considered less bothersome than scalp hair loss. Although itch was endorsed by 15 patients, only one did so spontaneously. Also, patients noted difficulty in differentiating itch related to disease and treatment versus itch related to other factors (e.g., application of AA treatments, wearing wigs). When discussing other signs and symptoms of AA, painful/sensitive skin was endorsed as a bothersome symptom but only by three participants when probed.
Participants also described the consequences of AA in their daily lives (Tables 4 and 5). Among the 20 adult participants, impacts on self-image (n = 12) as well as on social interactions and relationships (n = 12) were spontaneously endorsed by over half of participants. When probed, frustration was also endorsed by over half of the sample (n = 14) (Table 4). When spontaneous and probed reports were combined, the most frequent additional impacts reported were those affecting daily activities, including outdoor activities (n = 12), feelings of sadness (n = 11), and sexual function or intimacy (n = 11). When asked to identify the impact of AA that bothered them the most, adult participants reported altered self-image, emotional impacts, and impacts on social interactions and relationships.
Among adolescents (n = 7), impacts on participation in outdoor activities, often sports or water activities, were endorsed by nearly all participants (n = 6; Table 4). Impacts on self-image/feeling self-conscious were also endorsed by nearly all participants (n = 6). When probed, emotional impacts of feeling sad (n = 6), frustrated (n = 5), embarrassed (n = 5), and worried (n = 5) were endorsed by nearly all adolescents. When asked to identify the impact of AA that bothered them the most, adolescent participants endorsed impacts on physical activities as well as emotional impacts.
Across the adult and adolescent participants, similar consequences of AA emerged in spontaneous and probed reporting, including self-consciousness, impacts on social interactions, and frustration. Proportionally more adolescents than adults reported impacts relating to daily or outdoor activities; embarrassment, which was not separately probed among the adult participants, was endorsed by five of seven adolescent patients.
Review of Content Exploration Findings
Across the sources that informed content exploration, there was general consensus regarding the priority symptoms and impacts of AA. Table 6 provides an overview of concepts endorsed by five or more patients during CE interviews, and further supported by patient testimonials given during the FDA PFDD meeting, the qualitative literature, and existing COA measures. In addition to the hallmark symptom of scalp hair loss, activity limitations were consistently reported across sources, particularly those pertaining to social interactions and daily activities. Various emotional symptoms, including altered self-image, frustration, and sadness, were reported in multiple sources.
The AA consequence model is represented in Fig. 1 and incorporates information from the existing data sources and the qualitative patient interviews. The model further served to identify content for inclusion in an instrument representing clinical benefit from the patient perspective.
Alopecia Areata Consequence Model. The alopecia areata consequence model was developed iteratively. A literature and instrument review informed the initial list of concepts for the model, which was updated based on results from qualitative interviews with adults and adolescents with alopecia areata. Concepts with a green box correspond to items that were ultimately included in the Alopecia Areata Patient Priority Outcomes instrument
Review of the priority concepts alongside the existing AA measures confirmed that the existing instruments are missing concepts of priority to individuals with AA and/or employ response options and recall periods which are not likely to capture the impacts of disease and treatment in the context of an AA clinical trial. Based on this assessment, the team initiated de novo development of an AA-specific PRO measure.
Item Development
Based on concepts endorsed across the reviewed sources, six symptoms and seven impacts (a total of 13 concepts; Table 7) were selected as candidate items. Priority was given to concepts that were endorsed by a majority of CE participants and were issues that are able to be improved with treatment in the time frame of a clinical trial. Items were drafted employing a recall period and language reflective of how CE and CD participants described their experiences with AA.
Content Confirmation
A total of 16 patients participated in two iterative rounds (n = 8 in each round) of CD interviews: 9 adults (round 1, n = 5; round 2, n = 4) and 7 adolescents (round 1, n = 3; round 2, n = 4). The average participant age was 32 years for the adults and 15 years for the adolescents. Most participants were white (n = 15; 93.8%) and female (n = 10; 62.5%), with adults having been diagnosed with AA for an average of just under 15 years and adolescents for nearly 8 years. All participants had experienced a fixed period of hair loss of at least 6 months. Participants ranged in disease severity (Table 2).
Results and feedback were consistent across the adolescent and adult participants and thus are presented together. Response options for Items 1–4 on hair loss were changed from a numeric to a verbal response scale, and the wording of several items was modified to improve clarity. When reviewing the items, round 1 participants generally reported that the measure covered all relevant concepts and that no important concepts were missing. Items 5 and 6 (fingernail/toenail issues and itch) were not widely endorsed as part of patients’ experience with AA and were flagged for potential elimination.
Following the round 2 interviews, further refinements were implemented to yield the final item set. When queried about any concepts missing from the round 2 item set, participants generally reported that the items covered all relevant concepts. As expected, items addressing fingernail/toenail issues and itch were not widely endorsed and were thus removed from the final instrument content.
No participants reported difficulty with the contemporaneous recall period and understood that they were responding based upon their impression of their hair loss (scalp, eyebrow, eyelash, body) currently. Participants confirmed their ability to recall over the 1-week period for the remaining emotional symptoms and activity limitation items and that these item concepts were appropriate for assessment referencing a past-week time frame.
The 11 items in the final version of the measure, the Alopecia Areata Patient Priority Outcomes (AAPPO) (see Table 7), were interpreted consistently, deemed relevant, and easily understood and answered by interview participants. The CD interviews confirmed the content and led to refinements to the wording and response options of the final questionnaire.
Discussion
This study explored the consequences and priority treatment outcomes to individuals with AA, revealing the need for a novel PRO measure developed according to regulatory requirements and expectations for use in a clinical trial setting. Not surprisingly, the primary consequence of importance to individuals with AA was scalp hair loss. Additional consequences included hair loss from other areas (e.g., eyebrows), emotional impacts (e.g., sadness), and activity limitations (e.g., interactions with others).
Results from the CE interviews confirmed the relevance and priority of concepts identified in existing data sources and confirmed that no existing measure adequately captures the AA patient experience. Across adults and adolescents, the CE results indicate that the priority impacts of AA were relevant to both adult and adolescent participants. The priority concepts reported by adolescents with AA were consistent with those reported by adults with AA, indicating that a single measure is appropriate for both subgroups.
After two iterative rounds of CD interviews, both adolescent and adult participants found the items comprehensive, easy to understand, and simple to answer. Participants were able to respond using the specified recall periods. Participants did not identify any concepts relating to key impacts of AA that were missing from the item set, supporting the content validity of the measure. These results provide evidence that the final item set adequately and appropriately assesses priority consequences of AA to adolescents and adults.
Some limitations of this study should be noted. An important limitation is that adult participants in the CE interviews self-reported having a clinician diagnosis of AA, and clinical characteristics were not physician confirmed. However, clinical confirmation of diagnosis was an inclusion criterion for the CD portion of this study, and the priorities of the CE sample were acknowledged as relevant and comprehensive by the clinically confirmed CD participants. For both rounds of CD interviews, the researchers prioritized obtaining patients who had a confirmed diagnosis from an expert in dermatology, although this limited recruitment to a single clinical site. With 36 participants, the sample size of the study was small, although sufficient to accomplish the study objectives and achieve concept saturation. While CE and CD participants resided in five separate states, the overall geographic diversity of the patient sample was limited. An additional limitation is that CD interviews were conducted via telephone. Additional analyses are planned with a larger population to evaluate the AAPPO’s structure, scoring, and measurement properties and establish that it is a fit-for-purpose measure of the impacts of AA from the patient perspective.
Conclusions
The AAPPO instrument is a content-valid PRO measure that contains 11 items assessing hair loss and key impacts of importance to both adults and adolescents with AA. The AAPPO employs a contemporaneous recall period for the four hair loss items and a 1-week recall period (“over the past week”) for seven items addressing emotional consequences and activity limitations. Future research will evaluate the structure of the AAPPO, establish scoring and further support its use in AA clinical trials as a fit-for-purpose assessment of treatment benefit from the patient perspective. In addition, to enable use in global studies of AA, the measure should undergo linguistic validation for use in other countries and to ensure that its content is meaningful and representative of priority AA consequences to other cultures.
References
Skogberg G, Jackson S, Åstrand A. Mechanisms of tolerance and potential therapeutic interventions in alopecia areata. Pharmacol Ther. 2017;179:102–10.
Montgomery K, White C, Thompson A. A mixed methods survey of social anxiety, anxiety, depression and wig use in alopecia. BMJ Open. 2017;7:e015468.
Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397–403. https://doi.org/10.2147/CCID.S53985. (eCollection 2015)
Coondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. 2014;5(4):416–25. https://doi.org/10.4103/2229-5178.142483.
Food and Drug Administration. Patient-focused drug development: methods to identify what is important to patients. Draft guidance for industry, food and drug administration staff, and other stakeholders. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-methods-identify-what-important-patients-guidance-industry-food-and. Accessed 2 August 2020.
Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. 2009. https://www.fda.gov/downloads/drugs/guidances/UCM193282.pdf. Accessed 2 August 2018.
ePROVIDE. https://eprovide.mapi-trust.org/. Accessed 12 September 2019.
Food and Drug Administration. The voice of the patient: a series of reports from the U.S. Food and Drug Administration’s (FDA’s) patient-focused drug development initiative: Alopecia Areata. Meeting date: September 11, 2017; report date: 2018. https://www.fda.gov/media/112100/download. Accessed 21 February 2020.
Food and Drug Administration. The voice of the patient: a series of reports from the U.S. Food and Drug Administration’s (FDA’s) patient-focused drug development initiative: Alopecia Areata. Transcript date: September 11, 2017. https://www.fda.gov/media/110943/download. Accessed 21 February 2020.
Willis GB. Cognitive interviewing: a tool for improving questionnaire design. Thousand Oaks: Sage Publications; 2001.
Finlay AY, Khan GK. Dermatology life quality index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6.
Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133(11):1433–40.
Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5(2):105–10.
Nijsten TE, Sampogna F, Chren MM, Abeni DD. Testing and reducing skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126(6):1244–50.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83.
Ware JE Jr. The SF-36 health survey. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Philadelphia: Lippincott-Raven. 1996.
Mendoza TR, Osei JS, Shi Q, Duvic M. Development of the alopecia areata symptom impact scale. J Investig Dermatol Symp Proc. 2013;16(1):S51–2.
Endo Y, Miyachi Y, Arakawa A. Development of a disease-specific instrument to measure quality of life in patients with alopecia areata. Eur J Dermatol. 2012;22(4):531–6.
Fabbrocini G, Panariello L, De Vita V, et al. Quality of life in alopecia areata: a disease-specific questionnaire. J Eur Acad Dermatol Venereol. 2013;27(3):e276–81.
Acknowledgments
Funding
This study was developed under a research contract between RTI Health Solutions and Pfizer Inc. and was funded by Pfizer Inc. Pfizer Inc. also funded development of this article and the journal’s rapid service fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and Editorial Assistance
Kate Lothman of RTI Health Solutions provided medical writing services, which were funded by Pfizer Inc. Kathleen Wyrwich of Pfizer provided review and feedback on the draft manuscript.
Disclosures
Susan Martin and Nimanee Harris are employees of RTI Health Solutions and were paid consultants to Pfizer in connection with the development of this manuscript. Randall Winnette and Linda Deal are salaried employees of and hold stock shares and/or options in Pfizer Inc.
Compliance with Ethics Guidelines
The qualitative research study materials were reviewed and approved by the RTI Institutional Review Board (approval no. 14241). The study complied with the Declaration of Helsinki, and all interview participants provided informed consent.
Data Availability
The datasets generated and analyzed during the current study are not publicly available in order to protect participant confidentiality.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Winnette, R., Martin, S., Harris, N. et al. Development of the Alopecia Areata Patient Priority Outcomes Instrument: A Qualitative Study. Dermatol Ther (Heidelb) 11, 599–613 (2021). https://doi.org/10.1007/s13555-021-00508-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-021-00508-w