Abstract
Acne, one of the most common skin diseases, affects approximately 85% of the adolescent population, and occurs most prominently at skin sites with a high density of sebaceous glands such as the face, back, and chest. Although often considered a disease of teenagers, acne is occurring at an increasingly early age. Rosacea is a chronic facial inflammatory dermatosis characterized by flushing (or transient facial erythema), persistent central facial erythema, inflammatory papules/pustules, and telangiectasia. Both acne and rosacea have a multifactorial pathology that is incompletely understood. Increased sebum production, keratinocyte hyper-proliferation, inflammation, and altered bacterial colonization with Propionibacterium acnes are considered to be the underlying disease mechanisms in acne, while the multifactorial pathology of rosacea is thought to involve both vasoactive and neurocutaneous mechanisms. Several advances have taken place in the past decade in the research field of acne and rosacea, encompassing pathogenesis and epidemiology, as well as the development of new therapeutic interventions. In this article, we provide an overview of current perspectives on the pathogenesis and treatment of acne and rosacea, including a summary of findings from recent landmark pathophysiology studies considered to have important implications for future clinical practice. The advancement of our knowledge of the different pathways and regulatory mechanisms underlying acne and rosacea is thought to lead to further advances in the therapeutic pipeline for both conditions, ultimately providing a greater array of treatments to address gaps in current management practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acne
Acne is a chronic inflammatory disease of the pilosebaceous unit and occurs most prominently at skin sites with a high density of sebaceous glands [e.g., the face (99% of cases), back (60% of cases), and chest (15% of cases)] [1]. Although it predominantly affects the adolescent population (approximately 85%), it can also affect pre- and post-adolescents. The pathogenesis of acne is multifactorial and polymorphic, and several different grading systems have been developed to assess the severity of acne. Substantial acne is associated with social impairment, diminished quality of life, depression, and reduced global self-esteem [2, 3].
Acne Pathogenesis: New Horizons
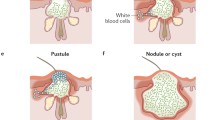
Several primary and secondary factors are believed to contribute to the onset and development of acne [4]. Specifically, the basic disease mechanism is thought to involve increased sebum production, keratinocyte hyperproliferation, inflammation, and altered bacterial colonization, primarily with Propionibacterium acnes (Fig. 1). The exact sequence of these events is unclear, but the major pathophysiologic factor is likely to be an androgen-induced increase in sebum production and secretion, coupled with qualitative changes in sebum. Characteristic changes in sebum composition reported in acne patients include reduced levels of linoleic acid, increased levels of squalene and lipid peroxides, and an increased ratio of saturated/mono-unsaturated fatty acids [4,5,6]. Hormones, the environment, neurologic and inflammatory mediators, and lipid metabolism have all been implicated in the regulation of sebum production [4].
The quantitative and qualitative changes in sebum production have also been implicated in colonization of the follicular duct by P. acnes. Notably, sebum quality may influence skin microbiome composition, particularly in terms of the abundance and strains of P. acnes populating the pilosebaceous unit. P. acnes is thought to contribute to acne pathogenesis through several different mechanisms including interaction with innate cutaneous immunity and keratinocyte and sebocyte function, leading to amplification of the three key pathologic processes implicated in acne development: inflammation, keratinization, and sebogenesis [7].
Support for the development of therapies that target molecules implicated in the activation of innate immunity is provided by several research findings. These include a confirmed association between sebaceous lipid synthesis and inflammation [8] and evidence of elevated levels of CD3+ and CD4+ T cells and inflammatory markers in early subclinical acne lesions (microcomedones) [9, 10]. Furthermore, recent studies highlight the important contributory role of Toll-like receptor activation and subsequent interleukin-1 alpha secretion by keratinocytes in comedogenesis [10, 11].
The pilosebaceous unit and resident sebocytes also play an active role in skin endocrine function. Androgen hormones as well as growth-promoting hormones and growth factors control sebaceous gland function, and recent attention has focused on insulin/insulin growth factor-1 signaling and its ability to stimulate sebocyte proliferation and differentiation. Importantly, endocrine changes closely related to pubertal rises in insulin resistance have been reported to affect acne onset and development, leading to a re-evaluation of nutritional influences and endocrine factors involved in the promotion of acne development [12]. The Western diet, characterized by a high glycemic load, may be an environmental factor linking acne to hyperinsulinemia and may represent a targetable adjunctive aspect of acne pathogenesis. A low-glycemic-load diet appears to ameliorate the signs of acne, reducing the number of both inflammatory and non-inflammatory lesions and affecting the fatty acid composition of sebum triglycerides through reduced fatty acid mono-unsaturation [4, 12]. Consumption of milk can induce mechanistic target of rapamycin-1 (mTORC1) signaling through several different pathways [13]. A major mechanism is considered to be the stimulation of IGF-1 production by the liver following ingestion of specific amino acids found in milk. These include tryptophan-rich lactalbumin, relevant for the hepatic synthesis of IGF-1, and the branched amino acids leucine, isoleucine, and valine, involved in the stimulation of insulin secretion [14]. Moreover, milk proteins possess approximately twice the amount of glutamine as beef, and glutamine in the sebaceous gland is required for cellular proliferation and lipogenesis, as a large amount is converted to the amino acids glutamate, alanine, serine, glycine, and aspartate [15]. A combination of these milk-derived metabolic effects can explain the elevated insulinemic index induced by the consumption of whole and skimmed milk. Evidence also suggests that peroxisome proliferator-activated receptors (PPAR) expressed in sebaceous-gland cells and their ligands play an important role in the regulation of human sebum production and acne development [8, 16, 17].
The clarification that sebum alterations and inflammation represent the primary events in acne pathogenesis indicates that these phenomena should be the primary therapeutic targets. In line with this view, systemic or topical antibiotic therapy should be prescribed for limited periods in patients with pustular or nodular lesions, whereas molecules to control sebum production and the inflammatory process should be prescribed longer term.
Focus on Pediatric Acne
The Earlier Onset of Acne
Although often considered a teenage disease, acne is occurring at an increasingly early age, possibly because of earlier puberty and/or other factors. Twelve years of age is no longer considered the low end of the ‘normal’ range for onset, and there has been an overall decrease in the average age of children seeking treatment for acne. This earlier onset mirrors a downward trend in age at the start of puberty and may represent the first sign of pubertal onset in children aged 7–11 years [18,19,20]. Acne and acne-like conditions can also develop in neonates, infants, and young children, and may be associated with differential diagnoses or systemic pathologies that differ from those of pre-teen and teenage acne vulgaris. The American Acne and Rosacea Society/American Academy of Pediatrics guidelines promote recognition of early acne, pathologic acne (acne associated with underlying endocrinologic or other pathologic conditions), and scarring acne [21].
Neonatal Acne
Neonatal acne develops during the first 0–6 weeks of life and is characterized by erythematous papulopustules affecting the face, scalp, neck, and torso. Not considered true acne, neonatal acne may be associated with skin colonization by Malassezia species (M. sympodialis, M. globosa) and is usually a self-limiting condition, although symptom resolution may be achieved more quickly with a topical anti-yeast cream [21, 22].
Infantile Acne
The term 'infantile acne' is given to acne that develops during the early months or first year of life. Comedones are usually present, often with papules, pustules, cysts, nodules, and scarring. Use of topical (benzoyl peroxide, retinoids, antibiotics) or systemic therapy (oral antibiotics and, in some circumstances, isotretinoin) has been reported in the literature and some guidelines [21]. The etiology of infantile acne is thought to be multifactorial, involving increased sebum excretion, stimulation of sebaceous glands by maternal or neonatal androgens, and colonization of sebaceous glands by Malassezia species [23].
Mid-Childhood Acne
Mid-childhood acne is very uncommon and affects children aged 1–7 years; a diagnosis warrants endocrinologic evaluation by a pediatric endocrinologist for causes of hyperandrogenism. It may be associated with premature adrenarche, Cushing’s syndrome, congenital adrenal hyperplasia, gonadal/adrenal tumors, or precocious puberty. Patient evaluation should also include assessment of growth, bone age, and Tanner stage and measurement of total/free testosterone, dehydroepiandrosterone, androstenedione, luteinizing hormone, follicle-stimulating hormone, prolactin, and 17-hydroxyprogesterone [21].
Pre-Adolescent Acne
Pre-adolescent acne (onset aged 7–12 years) is common and may precede other signs of pubertal maturation [21]. Investigation other than a medical history and physical examination is generally unnecessary unless there are signs of androgen excess, polycystic ovarian syndrome, or other systemic abnormalities. Pre-adolescent acne is characterized by the presence of comedones most frequently on the forehead and mid-face (rarely the trunk area) and an increase in sebum production and sebaceous follicle number [24,25,26].
In the US, physicians prescribe a wide variety of medications to treat pre-adolescent acne, and prescribing patterns vary substantially between clinicians of different specialties [27]. Shortcomings of current treatment approaches include over-reliance on oral antibiotics and underuse of topical retinoids, as well as prescribing of oral antibiotics without benzoyl peroxide or retinoids. Furthermore, there are ‘practice gaps’ (differences between practitioner prescribing and expert/guideline best practice recommendations) and a general under-appreciation of early, significant acne as a predictor of more severe acne over time.
Adolescent Acne
Adolescent acne manifests between the ages of 12 and 18 years, and is very common. According to the American Acne and Rosacea Society, treatment should be selected based on disease severity (mild, moderate, or severe) and the psychologic impact of the disease on the patient, including the likelihood of scarring and/or dyspigmentation. Treatment options include oral/topical antibiotic therapy, oral/topical retinoids, and benzoyl peroxide (Fig. 2) [21].
American Acne and Rosacea Society treatment recommendations for mild, moderate, and severe pediatric acne [21] (please refer to your local prescribing information for country-specific guidance). Reproduced with permission from Pediatrics, 131 Suppl 3, S163–6, Copyright © 2013 by the AAP. *Topical fixed-combination prescriptions available. **Assess adherence. †Consider dermatology referral
Treatment Challenges in Pediatric and Adolescent Acne
Special considerations in the treatment of pediatric patients with acne include previous treatment, cost, vehicle selection, ease of use, management of expectations and side effects, psychosocial impact on the patient, active scarring, and regimen complexity. In recent years, intensive antibiotic use has contributed to the development of antimicrobial resistance, with the emergence of antibiotic-resistant P. acnes and staphylococci strains becoming a major global health concern [28]. In an effort to address this, the new American Academy of Dermatology and European Dermatology Forum guidelines stipulate that systemic antibiotic use should be limited to the shortest possible duration, typically 3 months, and recommend against systemic antibiotic monotherapy [1, 21]. Furthermore, due to limited supportive data, the use of systemic antibiotics, other than tetracyclines and macrolides, is not recommended [29].
Hormonal therapy, in the form of combined oral contraceptives (COC), may be useful as second-line therapy for pubertal females with moderate/severe acne [21]. If considered an appropriate option, the patient’s tobacco use and family history of thrombotic events should be assessed, and initiation of COC therapy should be delayed until at least 1 year after onset of menstruation because of concerns about growth and bone density. Isotretinoin is recommended for severe, scarring, and/or refractory acne in adolescents and may be used in younger patients [21]; however, extensive counseling, particularly on avoiding pregnancy, and careful monitoring of potential side effects and toxicities are recommended for patients prescribed this treatment.
Given the concerns surrounding the emergence of drug resistance among patients with acne, drug development programs are moving away from oral antibiotic therapies and toward novel therapeutic approaches. Promising avenues of research currently include nitric oxide-based medications, drugs that target acetyl-CoA carboxylase, sebum or lipid synthesis mediators or inflammatory mediators, novel retinoids, and vaccines against P. acnes.
Rosacea
Rosacea is a chronic facial inflammatory dermatosis with manifold manifestations characterized by the presence of one or more of the following primary features: flushing (or transient facial erythema), persistent central facial erythema, inflammatory papules/pustules, and telangiectasia (for comprehensive reviews on rosacea, see [30, 31]). In addition, secondary features may be present, including: burning/stinging, plaque, dry appearance, edema, ocular manifestations, peripheral location, and phymatous changes. Rosacea can be divided into four subtypes (erythemato-telangiectatic, papulopustular, phymatous, and ocular), with erythemato-telangiectatic rosacea being the most common [32]. There is a wide range in the estimated prevalence of rosacea (0.1–22%) likely due to differences in case definitions. To address this issue and to concord with case definitions described by the National Rosacea Society, a highly sensitive screening instrument, Rosascreen, was developed [33]. Using this tool, followed by dermatologist verification of cases, rosacea prevalence in Germany and Russia has been reported at 12.3% and 5.0%, respectively [34]. Further use of this instrument in epidemiologic research may provide more accurate prevalence estimates.
Although environmental factors contribute to the development of rosacea, there is also a strong genetic component (46%) [35]. In a genome-wide association study, a single nucleotide polymorphism, intergenic between HLA-DR and BTNL2, and three HLA alleles, all coding for MHC class II proteins, were significantly associated with rosacea [36]. However, the phenotypic presentation of rosacea was not stated. In addition, an association has been established between rosacea and several chronic systemic diseases including gastroesophageal reflux disease, hyperlipidemia, hypertension, metabolic diseases, cardiovascular diseases, diabetes, celiac disease, multiple sclerosis, rheumatoid arthritis, and glioma [37,38,39,40]; however, the pathophysiologic link between rosacea and these conditions remains to be elucidated.
Optimizing Clinical Care: Application of the Latest Research
Rosacea has a multifactorial pathology involving vasoactive and neurocutaneous mechanisms, as well as innate and adaptive immunity. Each of these factors contributes to the disease to a different extent in each individual (Fig. 3). Over the past decade, the management of rosacea has evolved from empiricism to rational selection based on disease pathogenesis. While standard measures, including avoidance of triggers, gentle cleansers, and moisturizers in combination with sun protection, may mitigate flares, control signs and symptoms in some patients, others will require more specific therapy.
In the past, treatments for rosacea have primarily been confined to therapies indicated for other conditions (e.g., beta-blockers for flushing, antibiotics for acne vulgaris). However, more recently, treatments have been specifically developed based on our evolving understanding of the pathogenesis of rosacea (Fig. 4). Currently available treatment options based on positive outcomes from randomized controlled trials include topical brimonidine or intense pulsed light (IPL) for background persistent erythema; topical metronidazole, azelaic acid, ivermectin, or oral doxycycline and isotretinoin for papulopustules of rosacea; and cyclosporine eye drops for ocular rosacea [47]. Consensus on the optimal treatment for phymatous rosacea has yet to be reached because of a lack of robust clinical trial data. A useful summary of findings for all evidence-based interventions for treating different manifestations of rosacea is provided in a recently published Cochrane review [48].
Innate and adaptive immune dysfunction in rosacea and potential therapeutic targets. The sequence of innate immune activation in rosacea starts with factors increasing keratinocyte transcription of pro-cathelicidin (including vitamin D activated by UV, UV itself, infection, injury, and other triggers to barrier disruption) [43] and the serine proteases of the KLK family, KLK5 and KLK7 (activation mediated by TLR-2, which is upregulated by environmental and microbial stimuli) [42]. This leads to the formation of LL-37 and other peptides that are inflammatory and angiogenic [44]. Mast cells are pivotal mediators of cathelicidin-initiated skin inflammation—amplifying inflammation, vasodilation, and generation of LL-37 [45]. Chemokine and cytokine signals interact to generate a Th1/Th17-polarized adaptive immune response in rosacea [46]. Increased amounts of serine proteases can activate TRP via upregulation and/or activation of protease-activated receptors. There is co-localization of mast cells with unmyelinated sensory nerves, blood vessels, and myofibroblasts in rosacea (not shown) [41]. Sites of potential therapeutic intervention in these pathways are shown. KLK kallikrein, LL-37 cathelicidin, Th1 type 1 T-helper, Th17 type 17 T-helper, TL Toll-like receptor, UV ultraviolet
Although the past decade has witnessed important advances in our understanding and management of rosacea, it is anticipated that the findings from recent landmark pathophysiology studies will have important implications for future clinical practice. For example, gene array analyses indicate that each rosacea subtype can be differentiated by a selective gene profile, suggesting that the pathomechanisms of the different subtypes may vary with respect to the molecular pathways involved [49]. Other promising avenues of research include the role of cathelicidin antimicrobial peptides in aberrant innate immune responses [44, 50], the role of mast cells as key mediators of cathelicidin-initiated inflammation in rosacea [45], characterization of inflammatory infiltrate and cytokine/chemokine profiles, including Th1/Th17 pathway activation [46], and elucidation of mediators and receptors involved in neurovascular and neuroimmune aspects of rosacea [49]. Based on these recent basic science insights, mast-cell-stabilizing agents, calcitonin-gene-related peptide, substance P, and transient receptor potential channel inhibitors may represent possible contenders for future therapeutic strategies to treat rosacea.
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
References
Nast A, Dreno B, Bettoli V, et al. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(Suppl 1):1–29.
Halvorsen JA, Stern RS, Dalgard F, Thoresen M, Bjertness E, Lien L. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363–70.
Misery L. Consequences of psychological distress in adolescents with acne. J Invest Dermatol. 2011;131:290–2.
Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–32.
Ottaviani M, Camera E, Picardo M. Lipid mediators in acne. Mediators Inflamm. 2010. doi:10.1155/2010/858176. Accessed 23 Dec 2016.
Zouboulis CC, Jourdan E, Picardo M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J Eur Acad Dermatol Venereol. 2014;28:527–32.
Beylot C, Auffret N, Poli F, et al. Propionibacterium acnes: an update on its role in the pathogenesis of acne. J Eur Acad Dermatol Venereol. 2014;28:271–8.
Alestas T, Ganceviciene R, Fimmel S, Muller-Decker K, Zouboulis CC. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med (Berl). 2006;84:75–87.
Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20–7.
Dreno B, Gollnick HP, Kang S, et al. Understanding innate immunity and inflammation in acne: implications for management. J Eur Acad Dermatol Venereol. 2015;29(Suppl 4):3–11.
Selway JL, Kurczab T, Kealey T, Langlands K. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
Smith RN, Braue A, Varigos GA, Mann NJ. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41–52.
Melnik BC, John SM, Schmitz G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr J. 2013;12:103.
Hoyt G, Hickey MS, Cordain L. Dissociation of the glycaemic and insulinaemic responses to whole and skimmed milk. Br J Nutr. 2005;93:175–7.
Downie MM, Kealey T. Human sebaceous glands engage in aerobic glycolysis and glutaminolysis. Br J Dermatol. 2004;151:320–7.
Chen W, Yang CC, Sheu HM, Seltmann H, Zouboulis CC. Expression of peroxisome proliferator-activated receptor and CCAAT/enhancer binding protein transcription factors in cultured human sebocytes. J Invest Dermatol. 2003;121:441–7.
Makrantonaki E, Zouboulis CC. Testosterone metabolism to 5alpha-dihydrotestosterone and synthesis of sebaceous lipids is regulated by the peroxisome proliferator-activated receptor ligand linoleic acid in human sebocytes. Br J Dermatol. 2007;156:428–32.
Friedlander SF, Eichenfield LF, Fowler JF Jr, Fried RG, Levy ML, Webster GF. Acne epidemiology and pathophysiology. Semin Cutan Med Surg. 2010;29:2–4.
Goldberg JL, Dabade TS, Davis SA, Feldman SR, Krowchuk DP, Fleischer AB. Changing age of acne vulgaris visits: another sign of earlier puberty? Pediatr Dermatol. 2011;28:645–8.
Mancini AJ, Baldwin HE, Eichenfield LF, Friedlander SF, Yan AC. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30:S2–5.
Eichenfield LF, Krakowski AC, Piggott C, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. 2013;131(Suppl 3):S163–86.
Ayhan M, Sancak B, Karaduman A, Arikan S, Sahin S. Colonization of neonate skin by Malassezia species: relationship with neonatal cephalic pustulosis. J Am Acad Dermatol. 2007;57:1012–8.
Serna-Tamayo C, Janniger CK, Micali G, Schwartz RA. Neonatal and infantile acne vulgaris: an update. Cutis. 2014;94:13–6.
Lucky AW, Biro FM, Huster GA, Morrison JA, Elder N. Acne vulgaris in early adolescent boys. Correlations with pubertal maturation and age. Arch Dermatol. 1991;127:210–6.
Lucky AW, Biro FM, Huster GA, Leach AD, Morrison JA, Ratterman J. Acne vulgaris in premenarchal girls. An early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308–14.
Mourelatos K, Eady EA, Cunliffe WJ, Clark SM, Cove JH. Temporal changes in sebum excretion and propionibacterial colonization in preadolescent children with and without acne. Br J Dermatol. 2007;156:22–31.
Davis SA, Sandoval LF, Gustafson CJ, Feldman SR, Cordoro KM. Treatment of preadolescent acne in the United States: an analysis of nationally representative data. Pediatr Dermatol. 2013;30:689–94.
Leccia MT, Auffret N, Poli F, Claudel JP, Corvec S, Dreno B. Topical acne treatments in Europe and the issue of antimicrobial resistance. J Eur Acad Dermatol Venereol. 2015;29:1485–92.
Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945–73.
Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part II. Topical and systemic therapies in the treatment of rosacea. J Am Acad Dermatol. 2015;72:761–70.
Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015;72:749–58.
Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584–7.
Tan J, Leyden J, Cribier B, Audibert F, Kerrouche N, Berg M. Development and evaluation of a rosacea screening instrument (Rosascreen). J Cutan Med Surg. 2016;20:317–22.
Tan J, Schofer H, Araviiskaia E, Audibert F, Kerrouche N, Berg M. Prevalence of rosacea in the general population of Germany and Russia—the RISE study. J Eur Acad Dermatol Venereol. 2016;30:428–34.
Aldrich N, Gerstenblith M, Fu P, et al. Genetic vs environmental factors that correlate with rosacea: a cohort-based survey of twins. JAMA Dermatol. 2015;151:1213–9.
Chang AL, Raber I, Xu J, et al. Assessment of the genetic basis of rosacea by genome-wide association study. J Invest Dermatol. 2015;135:1548–55.
Rainer BM, Fischer AH, Luz Felipe da Silva D, Kang S, Chien AL. Rosacea is associated with chronic systemic diseases in a skin severity-dependent manner: results of a case-control study. J Am Acad Dermatol. 2015;73:604–8.
Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249–54.
Egeberg A, Hansen PR, Gislason GH, Thyssen JP. Clustering of autoimmune diseases in patients with rosacea. J Am Acad Dermatol. 2016;74:667–72.
Egeberg A, Hansen PR, Gislason GH, Thyssen JP. Association of rosacea with risk for glioma in a Danish nationwide cohort study. JAMA Dermatol. 2016;152:541–5.
Sulk M, Seeliger S, Aubert J, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol. 2012;132:1253–62.
Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688–97.
Yamasaki K, Schauber J, Coda A, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–80.
Yamasaki K, Di NA, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80.
Muto Y, Wang Z, Vanderberghe M, Two A, Gallo RL, Di Nardo A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol. 2014;134:2728–36.
Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198–208.
Schechter BA, Katz RS, Friedman LS. Efficacy of topical cyclosporine for the treatment of ocular rosacea. Adv Ther. 2009;26:651–9.
van Zuuren EJ, Fedorowicz Z, Carter B, van der Linden MM, Charland L. Interventions for rosacea. Cochrane Database Syst Rev. 2015;4:CD003262.
Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2–11.
Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12–5.
Acknowledgements
Sponsorship and article processing charges for this supplement were funded by Almirall S.A. This article is based on presentations from the 9th Skin Academy Symposium, 9–10 April, 2016, Barcelona, Spain, sponsored by Almirall S.A. All named authors meet the criteria of the International Committee of Medical Journal Editors (ICMJE) for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Figure 1: Image provided courtesy of Mauro Picardo with full patient consent. Medical writing support was provided by Chrissie Kouremenou of Complete Medical Communications, funded by Almirall S.A.
Disclosures
Mauro Picardo has received research grants from Angelini S.p.A., Cantabria Pharma, Colgate-Palmolive S.p.A., PPM Services S.A., and Fidia Farmaceutici S.p.a. Lawrence F. Eichenfield has been an advisor, investigator, and/or consultant for, and has received grants and/or honoraria from, Almirall S.A., Allergan, Galderma, Stiefel/GSK, Novan, and Valeant. Jerry Tan has been an advisor, consultant, investigator, and/or speaker and has received grants and/or honoraria from Almirall S.A., Allergan, Cipher, Dermira, Galderma, Stiefel/GSK, and Valeant.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/6C47F0600685C21C.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Picardo, M., Eichenfield, L.F. & Tan, J. Acne and Rosacea. Dermatol Ther (Heidelb) 7 (Suppl 1), 43–52 (2017). https://doi.org/10.1007/s13555-016-0168-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-016-0168-8