Abstract
Introduction
Propionibacterium acnes, a ubiquitous skin bacterium, stimulates keratinocytes to produce a number of proinflammatory cytokines and may contribute to inflammatory acne. The aim of the study was to investigate whether P. acnes-induced proinflammatory cytokine release is mediated by P. acnes-induced activation of p38 mitogen-activated protein kinase (p38 MAPK or p38) in human keratinocytes.
Methods
Immunohistochemistry was used to evaluate p38 phosphorylation in human skin samples with or without acne. Primary human keratinocytes and epidermal skin equivalents were exposed to viable P. acnes. Phosphorylation of MAPKs without or with p38 inhibitors was examined by Western blot and cytokine secretion was detected by Enzyme-Linked Immunosorbent Assay (ELISA).
Results
Increased levels of phospho-p38 were observed in human acne lesions, predominantly in follicular and perifollicular keratinocytes. Exposure of cultured human keratinocytes to viable P. acnes resulted in phosphorylation of multiple members of the MAPK family, including rapid and transient activation of p38 and extracellular signal-related kinase (ERK1/2) and relatively slow but sustained activation of c-Jun N-terminal kinases (JNK1/2). Viable P. acnes induced the secretion of interleukin-1α (IL-1α), tumor necrosis factor-α (TNF-α), and IL-8 from human keratinocytes. The phosphorylation of p38 (phospho-p38) and the secretion of cytokines induced by P. acnes in cultured keratinocytes were inhibited by SB203580, a p38α/β inhibitor. Furthermore, SCIO-469, a selective inhibitor of p38α, showed similar effects in cultured keratinocytes. Topical treatment of SCIO-469 inhibited the P. acnes-induced phospho-p38 and cytokine secretion in human epidermal equivalents.
Conclusion
The data demonstrate that P. acnes induces p38-dependent inflammatory responses in keratinocytes, and suggest that p38 may play an important role in the pathogenesis of inflammatory acne.
Funding
Johnson & Johnson.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Propionibacterium acnes contributes to inflammatory acne by stimulating keratinocytes, sebocytes, and immune cells to produce multiple proinflammatory cytokines such as interleukin (IL)-1α, tumor necrosis factor (TNF)-α, and chemokines (e.g., IL-8), which, in turn, attract neutrophils and mononuclear cells to the pilosebaceous unit [1–8]. P. acnes can also directly cause tissue injury [9–13]. In addition, P. acnes can activate inflammasomes and induce IL-1β secretion from monocytes [14, 15]. Toll-like receptors (TLRs) and nuclear factor kappa B (NF-κB) are involved in P. acnes-induced inflammation [4, 16–19]. Microbial products and cytokines act through the Toll-like, IL-1, and TNF receptor families to activate mitogen-activated protein kinases (MAPKs), leading to the activation of NF-κB [20–25]. Very few studies have investigated which MAPK may mediate the proinflammatory response [26, 27].
There are three major groups of MAPKs: the extracellular signal-related kinases (ERK1/2), the c-Jun N-terminal kinases (JNK1/2), and the p38 MAPKs (α, β, γ, and \( \delta \)) [20, 28–36]. ERKs are predominantly activated by mitogenic factors, while JNK and p38 are preferentially activated by proinflammatory cytokines and stress-inducing stimuli [33]. For p38 MAPKs, α and β are ubiquitously expressed, whereas γ and \( \delta \) are expressed in more restricted patterns [37]. In human keratinocytes, p38α and β are involved in responses to stress and proinflammatory cytokines, while p38\( \delta \) has been implicated in keratinocyte differentiation [38]. p38 MAPKs have been implicated in inflammatory disease pathways such as psoriasis, asthma, and Crohn’s disease and the p38α kinase might play a central role in inflammation [35, 39].
Inhibitors of p38 serve as valuable tools for elucidating the role of that enzyme in inflammatory responses. SB203580 is an inflammatory cytokine inhibitor that blocks both p38α and p38β kinase activities [37]. SCIO-469 is a relatively selective inhibitor of the α isoform of p38 [40]. Topical administration of SCIO-469 reduced acute skin inflammation in guinea pigs [41].
p38 regulates the production of inflammatory mediators that stimulate leukocyte recruitment and activation [42]. Many of these mediators (e.g., IL-1, TNF-α, and IL-8) are secreted by keratinocytes in response to P. acnes [5–7]. Thus, the purpose of this study was to evaluate whether the P. acnes-induced proinflammatory cytokine release is mediated by P. acnes-induced activation of p38 mitogen-activated protein kinase (p38 MAPK or p38) in human keratinocytes.
Methods
p38 Inhibitors
SCIO-469 was provided by Scios Inc. (Fremont, CA, USA) [40]. SB203580 was purchased from Sigma (St. Louis, MO, USA).
Cell Culture
Normal human neonatal epidermal keratinocytes were obtained from Cascade Biologics (Portland, OR, USA) and maintained in keratinocyte growth medium (KGM) (Cascade Biologics, Inc., Portland, OR, USA). All experiments were performed between passages 2–4 and at 70–80% cell confluence. KGM was used as antibiotic free in all the experiments described in this paper.
Propionibacterium acnes
Propionibacterium acnes strain ATCC 11828 was obtained from American Type Culture Collection (Manassas, VA, USA). Bacteria were grown for 48–72 h to reach stationary phase. Live bacteria were then harvested and re-suspended in KGM to achieve 108 CFU/mL for in vitro experiments.
Tissue Specimens
Five human acne lesional biopsies (three papules, one pustule, and one cystic acne) and five healthy scalp/neck skin were kindly provided by Drs. Jack L. Arbiser (Department of Dermatology, Emory University, Atlanta, GA, USA) and James J. Leyden (Department of Dermatology, University of Pennsylvania, PA, Philadelphia, USA) with informed consent. Five-micrometer-thick sections of biopsies were processed for immunostaining with rabbit anti-human phospho-p38 MAPK (Thr180/Tyr182) antibody (Cell Signaling Technology Inc., Danvers, MA, USA) [43].
The immunohistochemical assessment was done by visually assessing the intensity of the signal (brown color) in acne lesions vs healthy skin. A representative image of acne lesions was chosen with strong (dark brown color) nuclear staining of phospho-p38 and that of healthy skin was chosen with no/weak (no color/light brown color) nuclear staining of phospho-p38 in follicular and perifollicular epidermal keratinocytes.
Human Epidermal Equivalents
Human epidermal equivalents were purchased from MatTek Company (Asland, MA, USA). Upon receipt, human epidermal equivalents were incubated in EPI-100-ASY antibiotic-free MatTek assay medium overnight and then changed to KGM for another overnight incubation before topical treatment.
Enzyme-Linked Immunosorbent Assay (ELISA)
The protocol was adapted from Graham et al. [14] with modifications. In brief, human primary keratinocytes were pre-incubated for 1 h with either p38 inhibitors or with vehicle, and the treatments were stopped. The keratinocytes were then exposed to stationary-phase P. acnes (at a ratio of P. acnes to keratinocytes of 50:1) and were co-cultured for 16 h at 37 °C. As for human epidermal equivalents, they were topically treated with either vehicle or the p38 inhibitors for 2 h, followed by changing the medium to KGM in either absence or presence of live stationary-phase P. acnes at 108 CFU/mL for 16 h. After incubation, the supernatants were analyzed using Beadlyte® human multi-cytokine ELISA beadmaster™ Assays (Upstate Cell Signaling Solution, Lake Placid, NY, USA). Results are presented as the mean ± SD of eight replicate wells for keratinocytes or of two equivalents, in which triplicates were collected from each equivalent. Half-maximal inhibitory concentrations (IC50’s) were calculated using SigmaPlot Version 9.0 (Csystat Software Inc., San Jose, CA, USA).
Lactate dehydrogenase (LDH) assays (G-Biosciences, St. Louis, MO, USA) were performed in all the supernatants to ensure no cytotoxicity occurred in the cells under the experimental conditions.
Western Analysis
Activation of p38, ERK1/2, and JNK in human primary keratinocytes or epidermal equivalents was evaluated by Western blot using phospho-specific antibodies, and was normalized to the total level of the relevant proteins using anti-p38, anti-ERK1/2, and anti-JNK1/2 antibodies. Antibodies for phospho-p38 (Thr180/Tyr182), p38, phospho-p44/42 or ERK1/2 (Thr202/Tyr204), p44/42, phospho-SAPK/JNK (Thr183/Tyr185), SAPK/JNK, as well as goat anti-rabbit IgG HRP (horseradish peroxidase) and horse anti-mouse IgG HRP secondary antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). All other reagents for Western blot were purchased from Sigma (St. Louis, MO, USA), and blotting equipment was purchased from Bio-Rad (Hercules, CA, USA). The separated proteins were visualized by an ECL kit according to the manufacturer’s protocol (GE Healthcare Bio-Sciences Co., Piscataway, NJ, USA) and quantitated by densitometric analysis using a Kodak Gel Logic 100 Imaging System and Kodak 1D Imaging Software (Eastman Kodak Co., New Haven, CT, USA).
Statistics
Statistical analyses were performed using two-tailed Student’s t tests with unequal variances (Microsoft Office Excel 2007; Microsoft, Redmond, WA, USA). Differences were considered statistically significant if p < 0.05 (*), and highly significant if p < 0.01 (**) or p < 0.005 (***).
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Results
Activation of p38 in Human Acne Lesions
Phospho-p38 expression was detected in all five acne tissue samples with a nuclear localization. A representative image of healthy skin shows mainly diffuse cytoplasmic staining of phospho-p38 in both epidermal and dermal cells (Fig. 1a), and weak nuclear staining in some hair follicle cells (Fig. 1b). In contrast, markedly increased level of phospho-p38 was observed in the inflammatory acne lesion (Fig. 1c). The phospho-p38 was mostly confined to the nuclei of follicular and perifollicular epidermal keratinocytes (Fig. 1d).
p38 is activated in human acne lesions. a Healthy human skin shows diffuse cytoplasmic staining in both epidermis and dermis. b High magnification insert of a illustrates weak nuclear staining in some hair follicle cells of a healthy skin. c Strong nuclear staining of phospho-p38 is found in follicular and perifollicular epidermis and dermis of a representative human inflammatory acne lesion. d High magnification insert of c demonstrates a solid dot pattern of nuclear staining phospho-p38. Bar 100 µm
Propionibacterium acnes Induces a Time-Dependent Activation of MAPKs in Keratinocytes
Stationary-phase P. acnes stimulated rapid (within 5 min) phosphorylation of both p38 (phospho-p38) and ERK1/2 (phospho-ERK1/2), with peaks at 15 min (Fig. 2a). There was up to a sevenfold increase in phosphorylation of p38 and ERK1/2, compared to untreated (Fig. 2b, c). An additional activation peak of phospho-ERK1/2 occurred between 2 and 4 h (approximately sixfold increase; Fig. 2a, c). P. acnes also induced relatively slow but sustained phosphorylation of JNK1/2 (phospho-JNK1/2); the maximum increase was approximately fourfold between 2 and 8 h (Fig. 2a, d). These results show that viable P. acnes induced the activation of multiple MAPK pathways in cultured human keratinocytes.
Propionibacterium acnes induces activation of multiple MAPK pathways in cultured human keratinocytes. a Western blots show time-dependent induction of phospho-p38 (p-p38), phospho-ERK1/2 (p-ERK1/2) and phospho-JNK1/2 (p-JNK1/2). b–d The density of each phosphorylated MAPK band was normalized to its loading control, and then compared to time 0. MAPK mitogen-activated protein kinase, ERK extracellular signal-related kinases, JNK c-Jun N-terminal kinases
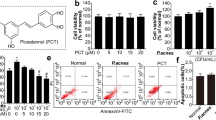
Propionibacterium acnes-Induced Phospho-p38 and Secretion of Cytokines are Suppressed by SB203580
Pretreatment of the keratinocytes with SB203580 at 10 and 25 μM reduced P. acnes-induced phospho-p38 to basal levels (Fig. 3a). Stationary-phase P. acnes stimulated the production of TNF-α (Fig. 3b) and IL-8 (Fig. 3c). Pretreatment of keratinocytes with SB203580 significantly suppressed P. acnes-induced secretion of TNF-α and IL-8 (Fig. 3b, c, both p < 0.005).
Propionibacterium acnes-induced phospho-p38 and cytokine release in keratinocyte cultures was inhibited by a p38α/β inhibitor, SB203580. a Western blots show that SB203580 inhibits P. acnes-induced phospho-p38. b–c SB203580 inhibits the secretion of TNF-α (b) and IL-8 (c). Data are expressed as mean ± SD. p < 0.05 (*), p < 0.01 (**), p < 0.005 (***). TNF tumor necrosis factor, PA Propionibacterium acnes, Veh vehicle
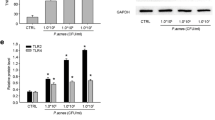
Propionibacterium acnes-Induced Activation of p38, but not ERK1/2 and JNK1/2, and Inflammatory Cytokine/Chemokine Secretion in Keratinocytes is Dose-Dependently Inhibited by SCIO-469
Pretreatment of the keratinocytes with SCIO-469 inhibited P. acnes-induced phospho-p38 in a dose-dependent fashion with a 50% inhibition at 16–80 nM, and complete inhibition was observed between 0.4 and 2 μM (Fig. 4a). There was no inhibition but upregulation of P. acnes induced either phospho-ERK1/2 or phospho-JNK1/2 (Fig. 4a). Stationary-phase P. acnes stimulated the production of TNF-α (Fig. 4b, p < 0.005), IL-8 (Fig. 4c, p < 0.005), and IL-1α (Fig. 4d, p < 0.005). Pretreatment of keratinocytes with SCIO-469 dose-dependently inhibited P. acnes-induced TNF-α secretion with an IC50 of 79 nM (Fig. 4b). SCIO-469 also significantly suppressed IL-8 and IL-1α secretion (Fig. 4c, d), but the degree of suppression was less for IL-8 and IL-1α than that observed for TNF-α.
Propionibacterium acnes-induced phosphorylation of p38, but not of ERK1/2 or JNK1/2, and the cytokine release was inhibited by a p38α inhibitor, SCIO-469, in human keratinocytes. a Western blots show that SCIO-469 inhibits P. acnes-induced phospho-p38 (p-p38) dose-dependently, but not of phospho-ERK1/2 (p-ERK1/2) or phospho-JNK1/2 (p-JNK1/2). b–c SCIO-469 inhibits the secretion of TNF-α (b), IL-8 (c), and IL-1α (d). Data are expressed as mean ± SD. p < 0.05 (*), p < 0.01 (**), p < 0.005 (***). ERK extracellular signal-related kinases, JNK c-Jun N-terminal kinases, TNF tumor necrosis factor, IL interleukin, PA Propionibacterium acnes
Topical SCIO-469 Inhibits P. acnes-Induced Phospho-p38 and Secretion of Cytokines in Human Epidermal Equivalents
Viable P. acnes induced both phospho-p38, and secretion of TNF-α and IL-8 from the equivalents, compared to the control without P. acnes (Veh-PA, Fig. 5a, b). Topical SCIO-469 at 4–100 μM inhibited P. acnes-induced phospho-p38 dose-dependently (Fig. 5a) as compared to P. acnes-induced plus vehicle-treated control (Veh + PA). In addition, topical SCIO-469 suppressed P. acnes-induced secretion of both TNF-α and IL-8 significantly (p < 0.005) at 20 and 100 μM (Fig. 5b, c). As expected, viable P. acnes also induced secretion of IL-1α in the skin equivalents. Inhibition of IL-1α by topical SCIO-469 was also observed, but with no obvious dose-response curve (data not shown).
Propionibacterium acnes-induced phospho-p38 and cytokine release was inhibited by a p38α inhibitor, SCIO-469, in human epidermal equivalents. a Western blot shows that SCIO-469 suppresses P. acnes-induced phospho-p38 (p-p38) dose-dependently. b–c SCIO-469 inhibits the secretion of TNF-α (b) and IL-8 (c). Data are expressed as mean ± SD. p < 0.05 (*), p < 0.01 (**), p < 0.005 (***). PA Propionibacterium acnes, TNF tumor necrosis factor, IL interleukin
Discussion
In this study, p38 MAPK was highly activated in the inflammatory acne lesions compared to normal skin. Using small molecule p38 inhibitors, it was further demonstrated that these compounds not only suppressed P. acnes-induced phosphorylation of p38 in vitro, but also decreased P. acnes-induced proinflammatory cytokine and chemokine production in keratinocytes in vitro. These data suggest that activation of p38 by P. acnes may be one of the contributors to the clinical development of inflammatory acne.
The current study was a pilot investigation on the histological and histochemical skin changes showing there was activation of p38 signaling in inflammatory acne lesions. A limitation of the current study was the small number of acne biopsies that could be obtained and evaluated. Obtaining biopsies of acne lesions was challenging which limits the extrapolation of the results. In addition, this study evaluated p38 only in inflammatory acne lesions; it could be interesting to examine p38 activation in early lesional samples such as comedones.
Exposure of keratinocytes to P. acnes induced the activation of multiple MAPK pathways. SB203580, a p38α/β inhibitor, inhibited P. acnes-induced phospho-p38. A second p38 pharmacological inhibitor, SCIO-469, specifically inhibited P. acnes-induced phospho-p38 in a dose-dependent fashion, suggesting the major isoform activated by P. acnes in keratinocytes is p38α. However, when phospho-p38 was blocked by SCIO-469, a concomitant upregulation of phospho-ERK1/2 and phospho-JNK1/2 was observed. This observation was consistent with previous findings [40, 44]. Similar data were also reported in a wound-healing model and a conditional p38α knock-out mouse model [45, 46].
Propionibacterium acnes stimulates the secretion of proinflammatory cytokines and chemokines such as IL-1, TNF-α, and IL-8 in cultured keratinocytes. IL-1α induces hypercornification of the infundibulum ex vivo, and was detected in the majority of open comedones of acne patients [47–49]. TNF-α may drive the early stage of inflammatory acne lesions [5]. IL-8 is a potent chemotactic agent for neutrophils, and both IL-8 and neutrophils are commonly found in high levels in inflammatory acne lesions [17, 50]. IL-8-induced chemotaxis of neutrophils may be activated by endogenous or exogenous (e.g., bacterial) factors to release lysosomal enzymes that can degrade the follicular epithelium, leading to further inflammation [51]. In lipopolysaccharide (LPS)-stimulated human monocytes, p38α MAPK was the molecular target for the biosynthesis of TNF-α and IL-1 [20, 21]. Data presented here not only confirm the increased production of TNF-α, IL-8, and IL-1 by keratinocytes upon stimulation with P. acnes, but also demonstrated the involvement of p38α MAPK in this process.
Moreover, the biopsies from clinically diagnosed inflammatory acne lesions showed a marked increase of phospho-p38 nuclear staining in follicular and perifollicular epidermal keratinocytes, suggesting translocation of activated p38 to the nuclei. The finding is similar to the localization of activated NK-κB and activator protein-1 (AP-1) in inflammatory acne lesions [17]. The proliferative effect could be due to the ability of P. acnes to activate p38 by stabilizing IL-8 mRNA, and subsequently to upregulate IL-8 secretion resulting in keratinocyte proliferation [52–55]. Both live and heat-killed P. acnes can induce phosphorylation of heat shock protein 27, a downstream cellular stress mediator of p38 [26]. Although our study only focused on acne lesional skin and non-lesional healthy skin, previous studies have reported that non-lesional acne skin adjacent to acne lesions (perilesional) showed overexpression of proinflammatory cytokines (IL-1α) and increased immune cells, but did not exhibit hyperproliferation or abnormal differentiation of the follicular epithelium [56]. It is interesting to speculate that the presence of inflammation alone is not sufficient to produce acne but perhaps, P. acnes-induced inflammatory signaling is an initiating event in comedone formation; however, to produce clinical acne lesions may require a number of P. acnes-induced signaling pathways in keratinocytes that affect inflammatory responses, cell proliferation, and hyperkeratinization.
Conclusion
This study shows an increase in phospho-p38 in biopsies of acne lesions predominantly in follicular and perifollicular keratinocytes. P. acnes induces inflammatory responses in cultured keratinocytes and skin epidermal equivalents. p38 MAP kinase inhibitors reduce P. acnes-induced inflammation in vitro. These findings, together with the existing evidence showing activation of TLRs and NFκB in acne lesions, indicate that multiple inflammatory signaling molecules are activated in keratinocytes upon stimulation with P. acnes and that cross-communication among these mediators may be crucial in the pathogenesis of acne.
References
Puhvel SM, Sakamoto M. Cytotoxin production by comedonal bacteria (Propionibacterium acnes, Propionibacterium granulosum and Staphylococcus epidermidis). J Invest Dermatol. 1980;74:36–9.
Webster GF, Leyden JJ. Characterization of serum-independent polymorphonuclear leukocyte chemotactic factors produced by Propionibacterium acnes. Inflammation. 1980;4:261–9.
Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158–65.
Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–41.
Graham GM, Farrar MD, Cruse-Sawyer JE, et al. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol. 2004;150:421–8.
Schaller M, Loewenstein M, Borelli C, et al. Induction of a chemoattractive proinflammatory cytokine response after stimulation of keratinocytes with Propionibacterium acnes and coproporphyrin III. Br J Dermatol. 2005;153:66–71.
Nagy I, Pivarcsi A, Koreck A, et al. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124:931–8.
Nagy I, Pivarcsi A, Kis K, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–205.
Puhvel SM, Reisner RM. The production of hyaluronidase (hyaluronate lyase) by Corynebacterium acnes. J Invest Dermatol. 1972;58:66–70.
Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977;6:555–8.
Scott DG, Cunliffe WJ, Gowland G. Activation of complement—a mechanism for the inflammation in acne. Br J Dermatol. 1979;101:315–20.
Webster GF, Leyden JJ, Nilsson UR. Complement activation in acne vulgaris: consumption of complement by comedones. Infect Immun. 1979;26:183–6.
Ingham E, Holland KT, Gowland G, et al. Purification and partial characterization of an acid phosphatase (EC 3.1.3.2) produced by Propionibacterium acnes. J Gen Microbiol. 1980;118:59–65.
Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134:310–3.
Qin M, Pirouz A, Kim M, et al. Propionibacterium acnes induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381–8.
Medzhitov R, Preston-Hurlburt P, Janeway CAJ. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7.
Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691–9.
Hatada EN, Krappmann D, Scheidereit C. NF-kappaB and the innate immune response. Curr Opin Immunol. 2000;12:52–8.
Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105–13.
Lee JC, Laydon JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–42.
Lee JC, Young PR. Role of CSBP/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leuko Biol. 1996;59:152–7.
Carter AB, Knudtson KL, Monick MM, et al. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J Biol Chem. 1999;274:30858–63.
Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69.
Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11.
Aggarwal BB. Signaling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;9:745–56.
Lyte P, Sur R, Nigam A, et al. Heat-killed Propionibacterium acnes is capable of inducing inflammatory responses in skin. Exp Dermatol. 2009;18:1070–2.
Lee W, Kim K, An H., et al. The protective effects of Melittin on Propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J Invest Dermatol. 2014. doi:10.1038/jid.2014.75.
Han J, Lee JD, Bibbs L, et al. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11.
Jiang Y, Chen C, Li Z, et al. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β). J Biol Chem. 1996;271:17920–6.
Lechner C, Zahalka MA, Giot JF, et al. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci USA. 1996;93:4355–9.
Li Z, Jiang Y, Ulevitch RJ, et al. The primary structure of p38γ: a new member of p38 group of MAP kinases. Biochem Biophys Res Commun. 1996;228:334–40.
Kumar S, McDonnell PC, Gum RJ, et al. Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem Biophys Res Commun. 1997;235:533–8.
Saklatvala J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr Opin Pharmacol. 2004;4:372–7.
Cuenda A, Rousseau S. p38 MAP-Kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–75.
Coulthard LR, White DE, Jones DL, et al. p38MAPK: stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369–79.
Plotnikov A, Zehorai E, Procaccia S, et al. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–33.
Adams JL, Badger AM, Kumar S, et al. p38 MAP kinase: molecular target for the inhibition of pro-inflammatory cytokines. Prog Med Chem. 2001;38:1–60.
Eckert RL, Efimova T, Balasubramanian S, et al. p38 mitogen-activated protein kinases on the body surface—a function for p38δ. J Investig Dermatol. 2003;120:823–8.
Schieven GL. The p38α kinase plays a central role in inflammation. Curr Topics Med Chem. 2009;9:1038–48.
Hideshima T, Podar K, Chauhan D, et al. p38 MAPK inhibition enhances PS-341 (bortezomib)-induced cytotoxicity against multiple myeloma cells. Oncogene. 2004;23:8766–76.
Medicherla S, Ma JY, Reddy M, et al. Topical alpha-selective p38 MAP kinase inhibition reduces acute skin inflammation in guinea pig. J Inflamm Res. 2010;3:9–16.
Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13.
Eisinger M, Li W, Anthonavage M, et al. A melanocortin receptor 1 and 5 antagonist inhibits sebaceous gland differentiation and the production of sebum-specific lipids. J Dermatol Sci. 2011;63:23–32.
Kim C, Sano Y, Todorova K, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–27.
Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003;278:21989–97.
Caballero-Franco C, Choo M, Sano Y, et al. Tuning of protein kinase circuitry by p38 is vital for epithelial tissue homeostasis. J Biol Chem. 2013;288:23788–97.
Guy R, Kealey T. The effects of inflammatory cytokines on the isolated human sebaceous infundibulum. J Invest Dermatol. 1998;110:410–5.
Downie MMT, Sanders DA, Kealey T. Modelling the remission of individual acne lesions in vitro. Br J Dermatol. 2002;147:869–78.
Ingham E, Eady EA, Goodwin CE, et al. Proinflammatory levels of interleukin-1 alpha-like bioactivity are present in the majority of open comedones in acne vulgaris. J Invest Dermatol. 1992;98:895–901.
Trivedi NR, Gilliland KL, Zhao W, et al. Gene array expression profiling in acne lesions reveals marked upregulation of gene involved in inflammation and matrix remodeling. J Invest Dermatol. 2006;126:1071–9.
Webster GF, Leyden JJ, Tsai CC, et al. Polymorphonuclear leukocyte lysosomal release in response to Propionibacterium acnes in vitro and its enhancement by sera from inflammatory acne patients. J Invest Dermatol. 1980;74:398–401.
Winzen R, Kracht M, Wilhelm A, et al. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–80.
Frevel MA, Bakheet T, Silva AM, et al. p38 Mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol Cell Biol. 2003;23:425–36.
Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–21.
Michel G, Kemeny L, Peter RU, et al. Interleukin-8 receptor-mediated chemotaxis of normal human epidermal cells. FEBS Lett. 1992;305:241–3.
Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20–7.
Acknowledgments
The authors gratefully acknowledge Kelly Dunn (Johnson & Johnson) for culturing and providing the P. acnes and Dr. Miri Seiberg for valuable discussions of this manuscript. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Sponsorship and article processing charges for this study were funded by Johnson & Johnson.
Conflict of interest
W. H. Li was an employee of The Johnson & Johnson Skin Research Center, Consumer & Personal Products Worldwide, Johnson & Johnson Family of Consumer Companies, Inc. when these experiments were conducted. L. Zhang was an employee of The Johnson & Johnson Skin Research Center, Consumer & Personal Products Worldwide, Johnson & Johnson Family of Consumer Companies, Inc. when these experiments were conducted. P. Lyte was an employee of The Johnson & Johnson Skin Research Center, Consumer & Personal Products Worldwide, Johnson & Johnson Family of Consumer Companies, Inc. when these experiments were conducted. K. Rodriguez was an employee of The Johnson & Johnson Skin Research Center, Consumer & Personal Products Worldwide, Johnson & Johnson Family of Consumer Companies, Inc. when these experiments were conducted. D. Cavender was an employee of The Johnson & Johnson Skin Research Center, Consumer & Personal Products Worldwide, Johnson & Johnson Family of Consumer Companies, Inc. when these experiments were conducted. M. D. Southall was an employee of The Johnson & Johnson Skin Research Center, Consumer & Personal Products Worldwide, Johnson & Johnson Family of Consumer Companies, Inc. when these experiments were conducted.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Li, WH., Zhang, L., Lyte, P. et al. p38 MAP Kinase Inhibition Reduces Propionibacterium acnes-Induced Inflammation in Vitro. Dermatol Ther (Heidelb) 5, 53–66 (2015). https://doi.org/10.1007/s13555-015-0072-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-015-0072-7