Abstract
Introduction
This study was conducted using an integrated retrospective database to evaluate the effectiveness of Omnitrope® (Sandoz) on children with growth hormone deficiency (GHD), idiopathic short stature (ISS), and Turner Syndrome (TS) who switched from a non-Omnitrope recombinant human growth hormone (rhGH) preparation during routine clinical care.
Methods
This was a retrospective study which identified patients with GHD, ISS, and TS during the study time period of January 1, 2006 and July 31, 2011. Patients were included if they switched to Omnitrope from another non-Omnitrope rhGH therapy during the study time period, were <18 years of age at time of switch, and on a prior rhGH therapy for at least 15 months pre-switch and on Omnitrope for 15 months post-switch. Auxological parameters (height, height standard deviation score [HSDS], height velocity [HV], and height velocity standard deviation score [HVSDS]) were evaluated during post-switch.
Results
One hundred and three patients were identified: GHD (n = 57), ISS (n = 26), and TS (n = 20). There was continuous growth in height for all 103 patients with an average rate of 6.52 cm over the 15-month post-switch period. Patients with GHD grew an average rate of 6.30 cm, patients with ISS grew an average rate of 6.58 cm, and patients with TS grew an average rate of 6.52 cm over the 15-month post-switch period. The average rate of HSDS was increased by 0.04 for all patients. The HV and HVSDS demonstrated the expected decline with advancing age and prolonged duration of treatment.
Conclusions
The growth trajectories of rhGH-treated patients were not negatively impacted by switching to Omnitrope and growth rates remained as expected prior to the switch.
Similar content being viewed by others
Introduction
There are currently nine recombinant human growth hormone (rhGH) products available in the USA for ten different indications [1]. Most of these rhGH products are approved for one or more indications. Growth hormone deficiency (GHD), idiopathic short stature (ISS), and Turner syndrome (TS) are some of the indications for which an rhGH is prescribed. Patients with GHD or ISS make up the majority of the pediatric population receiving growth hormone treatment [1]. GHD affects ~1 in 3,500 children [2]. In 2003, the Food and Drug Administration approved rhGH for children with ISS whose height is more than 2.25 standard deviations (SD) below the mean (or below the 1st percentile). Since the specific etiology for ISS in children is sometimes difficult to identify, children are often diagnosed with ISS and receive growth hormone (GH) therapy [3, 4]. TS occurs ~1 in 2,000–2,300 live female births [5].
Omnitrope® (Sandoz) is one of the rhGH products available in the USA. It was developed as a medicinal product similar to the reference rhGH product, Genotropin® (Pfizer Inc.). Long-term studies comparing Omnitrope and Genotropin have shown similar efficacy and safety [6–9]. Physicians are often confronted with the need to change the rhGH used by their patients due to health plan and/or patients’ insurance demands. Thus, there is a need for data demonstrating what impact switching rhGHs has on patients’ auxological measurements. During 2009, Kaiser Permanente Southern California (KPSC) had a formulary change which transitioned patients on other rhGHs to Omnitrope, thus providing a unique opportunity to evaluate the effects of switching preparations.
The primary objective of this study was to evaluate the effectiveness of Omnitrope on children with GHD, who switched from a non-Omnitrope rhGH preparation during routine clinical care. Secondary objectives were to evaluate the effectiveness of Omnitrope on children with an alternative diagnosis such as ISS or TS. This study considered quantitative outcomes evaluating change in auxological parameters such as height, height standard deviation score (HSDS), height velocity (HV), and height velocity standard deviation score (HVSDS) from time of their switch to Omnitrope until 15 months post-switch.
Methods
Setting
KPSC is a non-profit, group-model, health maintenance organization (health plan) providing integrated healthcare services to more than 3.6 million active members in Southern California, USA. The health plan covers the seven most populous counties in Southern California, from Los Angeles south to San Diego and east to the inland counties of Riverside and San Bernardino. KPSC membership closely mirrors the Southern California population, is racially diverse and includes the entire socioeconomic spectrum [10, 11]. Patient information on demographics and healthcare encounters (diagnoses, procedures, laboratory results, and prescriptions) are captured in the KPSC electronic medical record (eMR) system. KPSC members receive the majority of their healthcare and prescriptions at Kaiser Permanente facilities, which provides an ideal environment to conduct research studies.
Study Design and Patients
This retrospective cohort design study was conducted for the KPSC region only and was approved by the KPSC Institutional Review Board. Patients included in the study had GHD, ISS, or TS, and were switched to Omnitrope from another non-Omnitrope rhGH therapy between January 1, 2006 and July 31, 2011. We identified GHD, ISS, or TS by International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) of 253.xx (GHD), 783.xx (ISS), and 758.6 (TS). Each chart was then further reviewed to evaluate the diagnosis and code. Index date was defined as the date the patient switched from non-Omnitrope rhGH to Omnitrope. Pre-switch was defined as 15 months prior index date and post-switch as 15 months duration after the index date. Additional inclusion criteria were continuous membership and drug benefit eligibility in KPSC for 15 months pre-switch and 15 months post-switch; age <18 years on index date; and in receipt of a non-Omnitrope rhGH therapy for 15 months pre-switch. The 15-month pre-switch criteria were incorporated to limit potential bias associated with “catch-up growth” that is observed during initial GH therapy. The study observation period ended on October 31, 2012 so that each patient had a 15-month post-switch follow-up period. Patients had to have at least three documented visits that were 3 months apart in the 15 months pre-switch and at least three visits that were 3 months apart in the 15 months post-switch. The visits had to span more than 9 months before and after the index date. Once the initial cohorts were assembled, patients were further categorized according to their pubertal stage via Tanner staging [12, 13]. Patients with a Tanner stage of <2 were categorized as pre-pubertal and patients with Tanner stage of ≥2 categorized as pubertal.
Efficacy Assessments
The primary study outcomes were auxological changes in height, HSDS, HV, and HVSDS from the index date to 15 months post-switch. The height measurement closest to the index date was the baseline value. All patients had their baseline height value within 7 days prior to switching to Omnitrope. HV (cm/year) was calculated as the difference between two height measurements divided by the time interval between these two measurements multiplied by 365.25. Standardization of height used US Centers for Disease Control and Prevention reference ranges of body height and the mean and SD ranges of normally growing children [14] taken from tables provided by Tanner et al. [15]. Standardization of HV was based on the table provided by Tanner et al. [15]. We required three visits pre- and post-switch; when visits did not fall into the exact time position, we interpolated the data; we used the closest before and after height measurements to the time position of need and calculated the height point.
Statistical Analysis
Descriptive statistical analysis was performed using SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA). Continuous parameters were summarized using descriptive statistics including mean and SD. Categorical parameters were summarized using frequencies and percentages. We conducted a quasi-experimental analysis in which the subjects were evaluated pre- and post-switch to Omnitrope therapy.
Results
Patient Characteristics
A total of 103 patients were included in the study: 57 patients with GHD, 26 with ISS, and 20 with TS. Patient baseline clinical characteristics are summarized in Table 1. There were slightly more pre-pubertal patients than pubertal patients among the GHD and TS patients, whereas the opposite occurred among the ISS patients. Similarly, the pre-pubertal patients were younger among the GHD and TS groups than in the ISS children. The youngest patient was 4-year old with TS. The mean total dose of rhGH given to the patients was similar among the three groups of patients during the pre-pubertal and pubertal stages. The mean duration patients were on a non-Omnitrope rhGH therapy was 4.6 years (SD ±1.24 years, minimum 1.52 years, maximum 5.84 years).
Height
The mean overall height at index date was 137.76 ± 18.72 cm for all patients, and the mean height at 15 months post-switch for all the patients was 144.28 ± 18.52 cm, showing an increase of 6.52 cm (Table 2). Within each category, the subjects’ mean height at the index date was 137.37 ± 21.26 cm, 145.11 ± 14.75 cm, and 129.31 ± 10.68 cm for the patients diagnosed with GHD, ISS, and TS, respectively. After 15 months of Omnitrope treatment, those with GHD grew (on average) by 6.30 cm, those with ISS grew (on average) by 6.58 cm, and those with TS grew (on average) by 6.52 cm (Table 3). Height profiles of each participant during the 15-month pre- and post-switch period relative to the time of their index date are shown in Figs. 1 (all patients) and 2 (by indication).
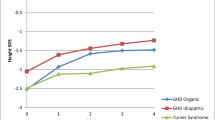
Height SDS
The mean HSDS for all subjects at index date was −1.49 ± 1.01; HSDS increased by an average of 0.04 over the 15-month post-switch period (Table 2). Within each category, the subjects’ mean HSDS at the index date was −1.23 ± 1.06, −1.70 ± 0.80, and −1.97 ± 0.90 for the patients diagnosed with GHD, ISS, and TS, respectively. Those with GHD improved, on average, by 0.07; those with ISS improved, on average, by 0.13; patients with TS had a change of −0.15 (Table 3). Figure 3 shows individual HSDS profiles over time, and indicates little impact on HSDS after the switch to Omnitrope, consistent with maintenance of growth during post-switch.
Height Velocity
The mean HV for all patients at index date was 6.00 ± 1.93 cm/year. Over the 15-month post-switch period, mean HV decreased by 1.06 cm/year (Table 2). Within categories, the subjects’ mean HVs at the index date was 6.15 ± 2.13 cm/year, 5.90 ± 1.67 cm/year, and 5.71 ± 1.68 cm/year for the patients diagnosed with GHD, ISS, and TS, respectively. Those with GHD, ISS, and TS decelerated by 0.84 cm/year, 1.16 cm/year, and 1.62 cm/year, respectively (Table 3).
Height Velocity SDS
The mean HVSDS for all patients was 0.78 ± 2.90 over the 15-month post-switch period. During the initial 6 months, there was an increase for all patients in HVSDS (at 3 months HVSDS was 1.03 ± 2.86, at 6 months HVSDS was 1.16 ± 3.20); however, after 9 months the HVSDS for all patients declined slightly (Table 2; Fig. 4). This could be contributed to the advancing age. The patients mean HVSDS declined by an average of 0.52 over the 15-month post-switch period (Table 2; Fig. 4). The subjects’ mean body HVSDS at the index date within each category was 0.71 ± 3.08, 1.45 ± 2.56, and 0.13 ± 2.75 for the patients diagnosed with GHD, ISS and TS, respectively (Table 3; Fig. 4). Those with GHD declined, on average, by 0.33. Those with ISS declined, on average, by 0.59. Patients with TS declined, on average, by 0.85.
Discussion
This present study has used real-world retrospective data to evaluate the impact of patients switching from a non-Omnitrope rhGH to Omnitrope in an US integrated healthcare system. Our findings indicate that such a switch can be conducted without negatively impacting the growth trajectories of the treated patients. This applies to the overall study population and also when considering the indication-specific subgroups (GHD, TS, ISS).
Patients were on a non-Omnitrope rhGH therapy for a mean duration of 4.6 years (SD ±1.24 years, minimum 1.52 years, maximum 5.84 years) and it has been shown in previous studies [16–20] that administration of growth hormone to children with GHD or ISS results in marked acceleration in linear growth, mostly during the first years of treatment. After 4–10 years of treatment, the HSDS increase wanes and does not differ significantly from the predicted score in the absence of therapy [16–20]. In this study, patients continued to grow in height and HSDS in all categories from index to 15 months post-switch. Results are expected and understandable given the age, overall duration of GH treatment, and pubertal status of the study population [16–20]. The mean HV for all patients in the study was 6.00 ± 1.93 cm/year over the 15-month post-switch period and is consistent with other reports of patients at similar durations of treatment [16, 17]. We recognize that the GH therapy dose that was used in TS patients pre- and post-switch was less than the recommended dose of 0.375 mg/kg/week. Nonetheless, patients’ individual height profiles were maintained after the switch. The overall growth rate for all the patients was similar with the similar GH dosages, and this may be due to how we identified the diagnosis codes for each patient in GH therapy. However, we reviewed the chart for each patient and tried to ensure that the diagnosis was categorized correctly.
Physicians are often faced with the need to change rhGH therapy, due to health plan and/or changes to a patient’s insurance, and this may be a cause of concern. Previous studies that have examined the impact of switching rhGH products have focused on parameters such as physician attitudes or the administrative burden on clinics, with the suggestion that patient care may be negatively impacted [21]. Alternatively, a comparative analysis of data from phase 3 studies demonstrated that switching rhGH therapy (from Genotropin to Omnitrope) has no impact on efficacy or safety in children with GHD [22]. More recently, a study from Sweden showed that patients with a range of pediatric growth disturbances could be successfully switched from Genotropin to Omnitrope, with no negative impact on growth and no serious or unexpected adverse drug reactions [23].
A limitation of our study is the retrospective nature of the analyses. Nevertheless, reporting of real-world data is of great value. We cannot exclude the possibility of inaccurate entry of data in the eMR system, although this possibility is equally likely to have occurred pre- and post-switch. We also required three visits that were 3 months apart during pre- and post-switch; however, some visits did not fall into the exact time position. We interpolated the data for some of these time positions using the closest height measurements before and after the time position of need, and calculated the height point.
Conclusion
This study used real-world retrospective data to examine the impact of switching from a non-Omnitrope rhGH to Omnitrope. The study demonstrated that patients continued to grow without alteration in their growth trajectories and can therefore be switched from a non-Omnitrope rhGH to Omnitrope without any negative impact on their growth. Our findings should be a useful resource for physicians who are faced with the possibility of switching rhGH therapy.
References
Navarro R, Dunn J, Lee P, Owens G, Rapaport R. Translating clinical guidelines into practice: the effective and appropriate use of human growth hormone. AJMC. 2013;19:S281–9.
Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125:29–35.
Fuqua J, Kemp S. Human Growth Foundation Inc.: idiopathic short stature 2008. Available from http://www.hgfound.org/pdf%20files/Idiopathic%20Short%20Stature.pdf. Accessed Jan 3, 2014.
Dunkel L. Management of children with idiopathic short stature. Eur J Endocrinol. 2006;155:S35–8.
Hook EB, Warburton D. The distribution of chromosomal genotypes associated with Turner’s syndrome: livebirth prevalence rates and evidence for diminished fetal mortality and severity in genotypes associated with structural X abnormalities or mosaicism. Hum Genet. 1983;64:24–7.
López-Siguero J, Borrás Pérez MV, Balser S, Khan-Boluki J. Long-term safety and efficacy of the recombinant human growth hormone Omnitrope® in the treatment of Spanish growth hormone deficient children: results of a phase III study. Adv Ther. 2011;28:879–93.
Romer T, Peter F, Saenger P, et al. Efficacy and safety of a new ready-to-use recombinant human growth hormone solution. J Endocrinol Invest. 2007;30:578–89.
Romer T, Saenger P, Peter F, et al. Seven years of safety and efficacy of the recombinant human growth hormone Omnitrope in the treatment of growth hormone deficient children: results of a phase III study. Horm Res. 2009;72:359–69.
Peter F, Romer T, Koehler B, et al. 48 Months data of treatment with the rhGH Omnitrope® 5 mg/ml lyophilized formulation in growth hormone deficient children: efficacy and safety results. LWPES/ESPE 8th Joint Meeting, New York, September 9–12, 2009, Abstract.
Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41.
Derose SF, Contreras R, Coleman KJ, Koebnick C, Jacobsen SJ. Race and ethnicity data quality and imputation using US census data in an integrated health system: the Kaiser Permanente Southern California experience. Med Care Res Rev. 2013;2013(70):330–45.
Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23.
Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303.
Centers for Disease Control and Prevention. Growth charts. Available from http://www.cdc.gov/growthcharts/. Accessed Dec 2012.
Tanner JM, Whitehouse H, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity for British Children, 1965. Part II. Arch Dis Child. 1966;41:613–35.
Vance M, Mauras N. Growth hormone therapy in adults and children. NEJM. 1999;341:1206–16.
Loche S, Cambiaso P, Setzu S, et al. Final height after growth hormone therapy in non-growth-hormone deficient children with short stature. J Pediatr. 1994;125:196–200.
Hindmarsh PC, Brook CGD. Final height of short normal children treated with growth hormone. Lancet. 1996;348:13–6.
Bernasconi S, Street ME, Volta C, Mazzardo G. Final height in nongrowth hormone deficient children treated with growth hormone. Clin Endocrinol (Oxf). 1997;47:261–6.
Maes M, Lindberg A, Price DA, Albertsson-Wikland K, Ranke MB. Long term growth response to growth hormone therapy in prepubertal children with idiopathic growth hormone deficiencies: analysis of Kabi International Growth Study. In: Kabi International Growth Study biannual report. No. 11. Mannheim, Germany: J&J; 1994. p. 1526.
Nelson WW, Frear RS. Physician attitudes toward human growth hormone products. Am J Health Syst Pharm. 1999;56:51–6.
Romer T, Zabransky M, Walczak M, Szalecki M, Balser S. Effect of switching recombinant human growth hormone: comparative analysis of phase 3 clinical data. Biol Ther. 2011;1:5.
Flodmark CE, Lilja K, Woehling H, Järvholm K. Switching from originator to biosimilar human growth hormone using dialogue teamwork: single center experience from Sweden. Biol Ther. 2013;3:35–43.
Acknowledgments
This research study and article processing charges were funded by Sandoz Inc. Partial medical writing assistance in the preparation of this paper was provided by Tony Reardon of Spirit Medical Communications Ltd, and his time was funded by Sandoz Inc. The authors would like to thank River Koblick for his assistance to the KPSC team as a research associate. The authors would also like to thank Alisa Hencken for her assistance to the Sandoz Inc. team. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Conflict of interest
Dr. Saenger is a paid consultant to Sandoz Inc. Dr. Lifshitz is a paid consultant to Sandoz Inc. Dr. Rapaport is a paid consultant to Sandoz Inc. Dr. Frankel is an employee of Sandoz Inc. Dr. Woehling is an employee of Sandoz International GmbH. Dr. Muenzberg is an employee of Sandoz International GmbH. Dr. Nazia Rashid and Yi-Lin Wu do not have any conflicts to disclose.
Compliance with ethics guidelines
This retrospective cohort design study was conducted for the KPSC region only and was approved by the KPSC Institutional Review Board.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rashid, N., Saenger, P., Wu, YL. et al. Switching to Omnitrope® from Other Recombinant Human Growth Hormone Therapies: A Retrospective Study in an Integrated Healthcare System. Biol Ther 4, 27–39 (2014). https://doi.org/10.1007/s13554-014-0017-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13554-014-0017-1