Abstract
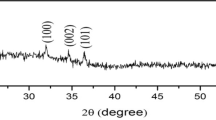
Zinc oxide thin films annealed at different temperatures (400, 450, 500, 550 °C) were prepared using the spray pyrolysis technique (SPT). X-ray scattering measurements have shown that the films were polycrystalline, having a hexagonal wurtzite structure with a dominated alignment in the (002) plane direction. The films undergo increases in crystallite size due to annealing temperatures. All the films showed more than 90% transparency across the whole visible spectrum. A blue shift of the visible energy gap was observed through increased annealing temperatures. Measurement of the gas response of the zinc oxide thin films to ethanol gas at an operating temperature of 150 °C and a gas concentration of 1000 ppm showed a high percentage response of 97.15%. Also, the response time and recovery time were measured for different annealing temperatures. The novelty in this research is the manufacture of a local system by the research team that can be used as a gas sensing system for thin films such as zinc oxide, which in turn succeeded in sensing ethanol gas.
Graphical Abstract








Similar content being viewed by others
References
K. Nakahara, H. Takasu, P. Fons, A. Yamada, K. Iwata, K. Matsubara, R. Hunger, S. Niki, Interactions between gallium and nitrogen dopants in ZnO films grown by radical-source molecular-beam epitaxy. Appl. Phys. Lett. 79(25), 4139–4141 (2001). https://doi.org/10.1063/1.1424066
J. Chen, T. Fujita, Effects of annealing on photoluminescence of ZnO thin film prepared by vapor phase growth. Jpn. J. Appl. Phys. 42(2R), 602 (2003). https://doi.org/10.1143/JJAP.42.602
H. Kim, C.M. Gilmore, J.S. Horwitz, A. Pique, H. Murata, G.P. Kushto, R. Schlaf, Z.H. Kafafi, D.B. Chrisey, Transparent conducting aluminum-doped zinc oxide thin films for organic light-emitting devices. Appl. Phys. Lett. 76(3), 259–261 (2000). https://doi.org/10.1063/1.125740
J. Wang, W. Chen, M. Wang, Properties analysis of Mn-doped ZnO piezoelectric films. J. Alloy. Compd. 449(1–2), 44–47 (2008). https://doi.org/10.1016/j.jallcom.2006.01.125
O.M. Abdulmunem, M.J. Mohammed Ali, E.S. Hassan, Optical and structural characterization of aluminium doped zinc oxide thin films prepared by thermal evaporation system. Optical Materials 109, 110374 (2020). https://doi.org/10.1016/j.optmat.2020.110374
T. Sergiu Shishiyanu, S. Shishiyanu Teodor, I. Lupan Oleg, Sensing characteristics of tin-doped ZnO thin films as NO2 gas sensor. Sensors and Actuators B: Chemical 107(1), 379–386 (2005). https://doi.org/10.1016/j.snb.2004.10.030
E. Suvaci, İ. Özgür Özer, Processing of textured zinc oxide varistors via templated grain growth. J. Eur. Ceram. Soc. 25(9), 1663–1673 (2005). https://doi.org/10.1016/j.jeurceramsoc.2004.05.026
S. Chakrabarti, and K. Dutta Binay. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Materials. 112(3), 269–278 (2004). https://doi.org/10.1016/j.jhazmat.2004.05.013
T. Meron, G. Markovich, Ferromagnetism in colloidal Mn2 -doped ZnO nanocrystals. J. Phys. Chem. B 109(43), 20232–20236 (2005). https://doi.org/10.1021/jp0539775
H. Michael Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo, P. Yang, Room-temperature ultraviolet nanowire nanolasers. Science 292(5523), 1897–1899 (2001). https://doi.org/10.1126/science.1060367
J. Koike, K. Shimoe, H. Ieki, 1.5 GHz low-loss surface acoustic wave filter using ZnO/sapphire substrate. Japan. J. Appl. Phys. 32(5S), 2337 (1993). https://doi.org/10.1143/JJAP.32.2337
J.B. Webb, D.F. Williams, M. Buchanan, Transparent and highly conductive films of ZnO prepared by rf reactive magnetron sputtering. Appl. Phys. Lett. 39(8), 640–642 (1981). https://doi.org/10.1063/1.92815
T. Minami, H. Nanto, S. Takata, Highly conductive and transparent aluminum doped zinc oxide thin films prepared by RF magnetron sputtering. Jpn. J. Appl. Phys. 23(5A), L280 (1984). https://doi.org/10.1143/JJAP.23.L280
M. Krunks, E. Mellikov, Zinc oxide thin films by the spray pyrolysis method. Thin Solid Films 270(1–2), 33–36 (1995). https://doi.org/10.1016/0040-6090(95)06893-7
K. Tominaga, T. Takao, A. Fukushima, T. Moriga, I. Nakabayashi, Amorphous ZnO–In2O3 transparent conductive films by simultaneous sputtering method of ZnO and In2O3 targets. Vacuum 66(3–4), 505–509 (2002). https://doi.org/10.1016/S0042-207X(02)00123-9
C. David Look, D.C. Reynolds, C.W. Litton, R.L. Jones, D.B. Eason, G. Cantwell, Characterization of homoepitaxial p-type ZnO grown by molecular beam epitaxy. Appl. Phys. Lett. 81(10), 1830–1832. https://doi.org/10.1063/1.1504875
N. Naghavi, C. Marcel, L. Dupont, A. Rougier, J. Bernard Leriche, C. Guéry, Structural and physical characterisation of transparent conducting pulsed laser deposited In2O3–ZnO thin films. J. Mater. Chem. 10(10), 2315–2319 (2000). https://doi.org/10.1039/B002094J
Y. Natsume, H. Sakata, Electrical and optical properties of zinc oxide films post-annealed in H2 after fabrication by sol–gel process. Mater. Chem. Phys. 78(1), 170–176 (2003). https://doi.org/10.1016/S0254-0584(02)00314-0
H. Jin Lim, D. Yong Lee, and Y. Jei Oh. Gas sensing properties of ZnO thin films prepared by microcontact printing. Sensors and Actuators A: Physical 125, no. 2 (2006): 405–410. https://doi.org/10.1016/j.sna.2005.08.031
S. Po Chang, and K. Yu Chen. UV illumination room-temperature ZnO nanoparticle ethanol gas sensors. ISRN Nanotechnology 2012 (2012). https://doi.org/10.5402/2012/453517
D. Zhang, G. Dong, Y. Cao, Y. Zhang, Ethanol gas sensing properties of lead sulfide quantum dots-decorated zinc oxide nanorods prepared by hydrothermal process combining with successive ionic-layer adsorption and reaction method. J Colloid Interface Sci. 15(528), 184–191 (2018). https://doi.org/10.1016/j.jcis.2018.05.085
J. Wu, D. Zhang, Y. Cao, Fabrication of iron-doped titanium dioxide quantum dots/molybdenum disulfide nanoflower for ethanol gas sensing. J. Colloid Interface Sci. 529, 556–567, ISSN 0021–9797 (2018). https://doi.org/10.1016/j.jcis.2018.06.049
M. Jiao, C.Nguyen Viet, N. Van Duy, H. Nguyen Duc, N. Van Hieu, K. Hjort, and H. Nguyen, Influence of annealing temperature on theperformance and stability of on-chip hydrothermally grown ZnO nanorod gassensor toward NO2. Acad. J. Sci. Res. 6(5), 180–189. https://doi.org/10.15413/ajsr.2018.0104
A. Katoch, G. Joo Sun, S. Woo Choi, J. Hyuk Byun, S. Sub Kim, Competitive influence of grain size and crystallinity on gas sensing performances of ZnO nanofibers. Sens. Actuators B: Chem. 185, 411–416 (2013). https://doi.org/10.1016/j.snb.2013.05.030
A. Katoch, Z. Ul Abideen, J. Hun Kim, S. Sub Kim, Crystallinity dependent gas-sensing abilities of ZnO hollow fibers. Met Mater Int 22(5), 942–946 (2016). https://doi.org/10.1007/s12540-016-6099-1
A. Bagheri Khatibani, Investigation of gas sensing property of zinc oxide thin films deposited by Sol-Gel method: effects of molarity and annealing temperature. Ind. J. Phys. 1–10 (2020). https://doi.org/10.1007/s12648-020-01689-4
S. Matsushima. T. Maekawa, J. Tamaki, N. Miura, N. Yamazoe, Role of additives on alcohol sensing by semiconductor gas sensor. Chem. Lett. 18(5), 845–848 (1989). https://doi.org/10.1246/cl.1989.845
A. Umar, R. Kumar, G. Kumar, H. Algarni, S.H. Kim, Effect of annealing temperature on the properties and photocatalytic efficiencies of ZnO nanoparticles. J. Alloy. Compd. 648, 46–52 (2015). https://doi.org/10.1016/j.jallcom.2015.04.236
T. Bora, K. Lakshman Karthik, S. Sarkar, A. Makhal, S. Sardar, K. Pal Samir, and J. Dutta, Modulation of defect-mediated energy transfer from ZnO nanoparticles for the photocatalytic degradation of bilirubin. Beilstein Journal of Nanotechnology 4(1), 714–725 (2013). https://doi.org/10.3762/bjnano.4.81
R. Kumar, G. Kumar, M.S. Akhtar, A. Umar, Sonophotocatalytic degradation of methyl orange using ZnO nano-aggregates. J. Alloy. Compd. 629, 167–172 (2015). https://doi.org/10.1016/j.jallcom.2014.12.232
A. Modwi, A. Ghanem Mohamed, M. Al-Mayouf Abdullah, A. Houas, Lowering energy band gap and enhancing photocatalytic properties of Cu/ZnO composite decorated by transition metals. J. Mole. Struct. 1173, 1–6 (2018). https://doi.org/10.1016/j.molstruc.2018.06.082
F. Abele’s, Optical properties of solids, North-Holland Publication co, New York (1972)
R. Swanepoel, Determination of thickness and optical constants of amorphous silicon. J. Phys. E: Sci. Instrum. 16, 1214–1222 (1983)
V.P. Deshpande, S.D. Sartale, A.N. Vyas, A.U. Ubale, Temperature dependent properties of spray deposited nanostructured ZnO thin films. Int J Mater Chem 7(2), 36–46 (2017). https://doi.org/10.5923/j.ijmc.20170702.03
D. Raoufi, T. Raoufi, The effect of heat treatment on the physical properties of sol–gel derived ZnO thin films. Appl. Surf. Sci. 255(11), 5812–5817 (2009). https://doi.org/10.1016/j.apsusc.2009.01.010
P.J. Scherrer, Estimation of the size and internal structure of colloidal particles by means of röntgen. Nachr. Ges. Wiss. Göttingen 2, 96–100 (1918). https://doi.org/10.1590/sajs.2013/a0019
E.S. Hassan, A.K. Elttayef, S.H. Mostafa, M.H. Salim, S.S. Chiad, Silver oxides nanoparticle in gas sensors applications. J. Mater. Sci. Mater. Electron. 30, 15943–15951 (2019). https://doi.org/10.1007/s10854-019-01954-1
D. Kohl, Surface processes in the detection of reducing gases with SnO2-based devices. Sensors and actuators 18(1), 71–113 (1989). https://doi.org/10.1016/0250-6874(89)87026-X
R.J.B. Balaguru, B.G. Jeyaprakash, Mimic of a gas sensor, metal oxide gas sensing mechanism, factors influencing the sensor performance and role of nanomaterials based gas sensors. NPTEL–Electrical & Electronics Engineering–Semiconductor Nanodevices (2004)
Acknowledgements
The authors would like to express their gratitude and acknowledge to the Department of Physics staff at College of Science of the Mustansiriyah University, the Laboratory of Advanced Materials, for making the necessary measurements.
Author information
Authors and Affiliations
Contributions
ES conceived the topic. OM and ES implemented and tested the samples. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Statement
Hereby, I Ehssan S. Hassan consciously assure that for the manuscript “Measuring the response of annealed zinc oxide thin films to ethanol gas” the following is fulfilled:
-
1.
The paper is not currently being considered for publication elsewhere.
-
2.
The paper reflects the authors' own research and analysis in a truthful and complete manner.
-
3.
The paper properly credits the meaningful contributions of co-authors and co-researchers.
-
4.
The results are appropriately placed in the context of prior and existing research.
-
5.
All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper reference.
-
6.
All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content.
-
7.
I agree with the above statements and declare that this submission follows the policies of Brazilian Journal of Physics Ionics as outlined in the Guide for Authors and in the Ethical Statement.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassan, E.S., Abdulmunem, O.M. Measuring the Response of Annealed Zinc Oxide Thin Films to Ethanol Gas. Braz J Phys 52, 160 (2022). https://doi.org/10.1007/s13538-022-01158-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13538-022-01158-9