Abstract
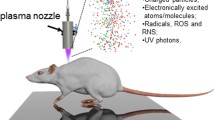
Wound healing involves a complex and dynamic interplay among various cell types, cytokines, and growth factors. Macrophages and transforming growth factor-β1 (TGF-β1) play an essential role in different phases of wound healing. Cold atmospheric plasma has a wide range of applications in the treatment of chronic wounds. Hence, we aimed to investigate the safety and efficacy of a custom-made plasma device in a full-thickness skin defect mouse model. Here, we investigated the wound tissue on days 6 and 12 using histology, qPCR, and western blotting. During the inflammation phase of wound repair, macrophages play an important role in the onset and resolution of inflammation, showing decreased F4/80 on day 6 of plasma treatment and increased TGF-β1 levels. The plasma-treated group showed better epidermal epithelialization, dermal fibrosis, collagen maturation, and reduced inflammation than the control group. Our findings revealed that floating electrode-dielectric barrier discharge (FE-DBD)-based atmospheric-pressure plasma promoted significantly faster wound healing in the plasma-treated group than that in the control group with untreated wounds. Hence, plasma treatment accelerated wound healing processes without noticeable side effects and suppressed pro-inflammatory genes, suggesting that FE-DBD-based plasma could be a potential therapeutic option for treating various wounds.




Similar content being viewed by others
References
Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2019;146:344–65. https://doi.org/10.1016/j.addr.2018.06.019.
Lee CR, Lee YJ, Kwon BY, Lee SJ, Ryu YH, Rhie JW, et al. Vessel-derived decellularized extracellular matrices (VdECM): novel bio-engineered materials for the Wound Healing. Tissue Eng Regen Med. 2023;20:59–67. https://doi.org/10.1007/s13770-022-00511-y.
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. https://doi.org/10.1152/physrev.00067.2017.
Gan J, Liu C, Li H, Wang S, Wang Z, Kang Z, et al. Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials. 2019;219:119340. https://doi.org/10.1016/j.biomaterials.2019.119340.
Sharifiaghdam M, Shaabani E, Faridi-Majidi R, De Smedt SC, Braeckmans K, Fraire JC. Macrophages as a therapeutic target to promote diabetic wound healing. Mol Ther. 2022;7(9):2891–908. https://doi.org/10.1016/j.ymthe.2022.07.016.
DiPietro LA, Wilgus TA, Koh TJ. Macrophages in healing wounds: paradoxes and paradigms. Int J Mol Sci. 2021;22:950. https://doi.org/10.3390/ijms22020950.
Moon S, Hong J, Go S, Kim BS. Immunomodulation for tissue repair and regeneration. Tissue Eng Regen Med. 2023;20:389–409. https://doi.org/10.1007/s13770-023-00525-0.
Fan MH, Zhu Q, Li HH, Ra HJ, Majumdar S, Gulick DL, et al. Fibroblast activation protein (FAP) accelerates collagen degradation and clearance from lungs in mice. J Biol Chem. 2016;291:8070–89. https://doi.org/10.1074/jbc.M115.701433.
Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Exp Rev Mol Med. 2011;13:e23. https://doi.org/10.1017/S1462399411001943.
Pyung YJ, Park DJ, Kim CG, Yun CH. Remodeling and restraining lung tissue damage through the regulation of respiratory immune responses. Tissue Eng Regen Med. 2023. https://doi.org/10.1007/s13770-022-00516-7.
Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994. https://doi.org/10.1016/0002-9610(94)90002-7.
Al Sadoun H. Macrophage phenotypes in normal and diabetic wound healing and therapeutic interventions. Cells. 2022;11:2430. https://doi.org/10.3390/cells11152430.
Feng X, Feng W, Ji Y, Jin T, Li J, Guo J. Transforming growth factor-β1 negatively regulates SOCS7 via EGR1 during wound healing. Cell Comm Signal. 2022;20:1–12. https://doi.org/10.1186/s12964-022-00893-5.
Dube CT, Ong YHB, Wemyss K, Krishnan S, Tan TJ, Janela B, et al. Age-related alterations in macrophage distribution and function are associated with delayed cutaneous wound healing. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.943159.
Kuninaka Y, Ishida Y, Ishigami A, Nosaka M, Matsuki J, Yasuda H, et al. Macrophage polarity and wound age determination. Sci Rep. 2022;12:20327. https://doi.org/10.1038/s41598-022-24577-9.
Braný D, Dvorská D, Halašová E, Škovierová H. Cold atmospheric plasma: a powerful tool for modern medicine. Int J Mol Sci. 2020;21:2932. https://doi.org/10.3390/ijms21082932.
Bernhardt T, Semmler ML, Schäfer M, Bekeschus S, Emmert S, Boeckmann L. Plasma medicine: Applications of cold atmospheric pressure plasma in dermatology. Oxi Med Cell Longev. 2019. https://doi.org/10.1155/2019/3873928.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CTmethod. Methods. 2001;25:402–8. https://doi.org/10.1006/meth.2001.1262.
Farhan M, Silva M, Xingan X, Zhou Z, Zheng W. Artemisinin inhibits the migration and invasion in uveal melanoma via inhibition of the PI3K/AKT/mTOR signaling pathway. Oxid Med Cell Logev. 2021. https://doi.org/10.1155/2021/9911537.
Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. https://doi.org/10.1016/j.immuni.2010.05.007.
Jiang T, Wang Z, Sun J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res Ther. 2020;11:1–10. https://doi.org/10.1186/s13287-020-01723-6.
Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, et al. Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review. Sys Rev. 2016;5:1–6. https://doi.org/10.1186/s13643-016-0329-y.
Boeckmann L, Schäfer M, Bernhardt T, Semmler ML, Jung O, Ojak G, et al. Cold atmospheric pressure plasma in wound healing and cancer treatment. Appl Sci. 2020;10:6898. https://doi.org/10.3390/app10196898.
Charipoor P, Nilforoushzadeh MA, Khani M, Nouri M, Ghasemi E, Amirkhani MA, et al. The FEDBD plasma’s quantitative investigation of skin parameters: skin elasticity, thickness, density, tissue oxygenation, perfusion, and edema. Heliyon. 2024;10(1):E23386. https://doi.org/10.1016/j.heliyon.2023.e23386.
Bong C, Choi JY, Bae J, Park S, Ko KS, Bak MS, et al. Effectiveness of ozone generated by a dielectric barrier discharge plasma reactor against multidrug-resistant pathogens and Clostridioides difficile spores. Sci Rep. 2022;12(1):14118. https://doi.org/10.1038/s41598-022-18428-w.
Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. https://doi.org/10.3389/fphys.2018.00419.
Komi DEA, Khomtchouk K, Santa Maria PL. A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms. Clin Rev Allergy Immunol. 2020;58:298–312. https://doi.org/10.1007/s12016-019-08729-w.
Johnson BZ, Stevenson AW, Prêle CM, Fear MW, Wood FM. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines. 2020;8:101. https://doi.org/10.3390/biomedicines8050101.
Abd El-Aleem SA, Abdelwahab S, AM‐Sherief H, Sayed A. Cellular and physiological upregulation of inducible nitric oxide synthase, arginase, and inducible cyclooxygenase in wound healing. J Cell Physiol. 2019;234:23618–32. https://doi.org/10.1002/jcp.28930.
Kim SY, Nair MG. Macrophages in wound healing: activation and plasticity. Immunol Cell Biol. 2019;97:258–67. https://doi.org/10.1111/imcb.12236.
Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and Wound Healing. Adv Wound Care (New Rochelle). 2012;1:10–6. https://doi.org/10.1089/wound.2011.0307.
Gao X, Lu C, Miao Y, Ren J, Cai X. Role of macrophage polarisation in skin wound healing. Int Wound J. 2023;20:2551–62. https://doi.org/10.1111/iwj.14119.
Sylvia C. The role of neutrophil apoptosis in influencing tissue repair. J Wound Care. 2003;12:13–6. https://doi.org/10.12968/jowc.2003.12.1.26458.
Lin PS, Chang HH, Yeh CY, Chang MC, Chan CP, Kuo HY, et al. Transforming growth factor beta 1 increases collagen content, and stimulates procollagen I and tissue inhibitor of metalloproteinase-1 production of dental pulp cells: role of MEK/ERK and activin receptor-like kinase-5/Smad signaling. J Formos Med Assoc. 2017;116:351–8. https://doi.org/10.1016/j.jfma.2016.07.014.
Yu H, Cheng W, Ding C, Li Z, Ouyang W, Liu Q, et al. A meaningful attempt: applying Dielectric Barrier Discharge plasma to Induce polarization of macrophages. Bioelectromagnetics. 2023;44:107–18. https://doi.org/10.1002/bem.22446.
Sharfuddin T, Jacho D, Mitchey D, Yildririm-Ayan E, Ayan H. A study on the effect of non-thermal plasma on macrophage phenotype modulation. Plasma Chem Plasma Process. 2023. https://doi.org/10.1007/s11090-023-10414-y.
Crompton RA, Williams H, Campbell L, Kheng LH, Saville C, Ansell DM, et al. An epidermal-specific role for arginase1 during cutaneous wound repair. J Invest Dermatol. 2022;142:1206–16. e1208.
Acknowledgements
The authors acknowledge Dr. Mohd Farhan for valuable discussions.
Funding
This work was partially supported by the Soonchunhyang University Research Fund and the National Research Foundation of Korea funded by the Korean government (MSIT) (grant numbers:2021R1I1A3048885, 2021-DD-RD-0480, and 2019R1A5A8083404).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent to publish
Hereby, all authors have approved the content for publication.
Ethical approval
This study does not involve human participants; hence ethical approval is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Subramaniam, M.D., Bae, J.S., Son, J. et al. Floating electrode–dielectric barrier discharge-based plasma promotes skin regeneration in a full-thickness skin defect mouse model. Biomed. Eng. Lett. 14, 605–616 (2024). https://doi.org/10.1007/s13534-024-00356-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-024-00356-5