Abstract
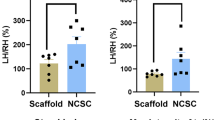
This study evaluated the biomechanical microenvironmental stimulating effect of pulsed electromagnetic field (PEMF) on the regeneration of crush-injured rat sciatic nerve, when combined with bone marrow mesenchymal stem cells (BMSCs) and recombinant human nerve growth factor (rhNGF-β), in the form of an adenoviral vector-mediated NGF. Sprague–Dawley rats were equally distributed into six groups; PBS, BMSC, NGF-Ad + BMSC, PEMF + PBS, PEMF + BMSC and PEMF + NGF-Ad + BMSC. The PBS group received PBS (volume: 10μL/rat), the BMSC group with BMSCs (1 × 106 cell/10 μL/rat) and NGF-Ad group with the rhNGF-β Ad infected BMSCs (1 × 106 cell/10 μL/rat) immediate after right sciatic nerve crush injury. The PEMF groups were exposed to PEMF of 1mT, 50 Hz, 1 h/day. The rats were observed for 3 weeks. PEMF alone did not showed the positive effect compared with negative control group. The groups transplanted with BMSCs showed higher axonal regeneration compared with the groups without transplantation of the cells whether BMSC was infected with NGF-Ad or not and whether the animals received PEMF. PEMF + NGF-Ad + BMSC group showed the significantly highest number of axons than the other groups. Functionally, all groups showed marked improvement at 3 weeks postoperatively although the difference was not statistically significant among the groups. PEMF showed the positive effect when combined with BMSC and NGF-ad in aspect of number of axons. Therefore, combining the microenvironment stimulation methods of PEMF and conventional methods such as transplantation of stem cells and growth factor could be considered for the regeneration methods in the nerve damage.





Similar content being viewed by others
References
Li R, Li DH, Zhang HY, Wang J, Li XK, Xiao J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol Sin. 2020;41:1289–300.
Moattari M, Kouchesfehani HM, Kaka G, Sadraie SH, Naghdi M. Evaluation of nerve growth factor (NGF) treated mesenchymal stem cells for recovery in neurotmesis model of peripheral nerve injury. J Craniomaxillofac Surg. 2018;46:898–904.
Abbas OL, Ozatik O, Gonen ZB, Kocman AE, Dag I, Ozatik FY, Bahar D, Musmul A. Bone marrow mesenchymal stem cell transplantation enhances nerve regeneration in a rat model of Hindlimb replantation. Plast Reconstr Surg. 2019;143:758e–68e.
Mert T, Gunay I, Gocmen C, Kaya M, Polat S. Regenerative effects of pulsed magnetic field on injured peripheral nerves. Altern Ther Health Med. 2006;12:42–9.
Walker JL, Kryscio R, Smith J, Pilla A, Sisken BF. Electromagnetic field treatment of nerve crush injury in a rat model: effect of signal configuration on functional recovery. Bioelectromagnetics. 2007;28:256–63.
Min Q, Parkinson DB, Dun XP. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia. 2021;69:235–54.
Li BH, Kim SM, Yoo SB, Kim MJ, Jahng JW, Lee JH. Recombinant human nerve growth factor (rhNGF-beta) gene transfer promotes regeneration of crush-injured mental nerve in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:e26-34.
Kim YT, Hei WH, Kim S, Seo YK, Kim SM, Jahng JW, Lee JH. Co-treatment effect of pulsed electromagnetic field (PEMF) with human dental pulp stromal cells and FK506 on the regeneration of crush injured rat sciatic nerve. Int J Neurosci. 2015;125:774–83.
Seo N, Lee SH, Ju KW, Woo J, Kim B, Kim S, Jahng JW, Lee JH. Low-frequency pulsed electromagnetic field pretreated bone marrow-derived mesenchymal stem cells promote the regeneration of crush-injured rat mental nerve. Neural Regen Res. 2018;13:145–53.
Sung MA, Jung HJ, Lee JW, Lee JY, Pang KM, Yoo SB, Alrashdan MS, Kim SM, Jahng JW, Lee JH. Human umbilical cord blood-derived mesenchymal stem cells promote regeneration of crush-injured rat sciatic nerves. Neural Regen Res. 2012;7:2018–27.
Jolicoeur FB, Rondeau DB, Hamel E, Butterworth RF, Barbeau A. Measurement of ataxia and related neurological signs in the laboratory rat. Can J Neurol Sci. 1979;6:209–15.
Bain J, Mackinnon S, Hunter D. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–38.
Wang T, Ito A, Aoyama T, Nakahara R, Nakahata A, Ji X, Zhang J, Kawai H, Kuroki H. Functional evaluation outcomes correlate with histomorphometric changes in the rat sciatic nerve crush injury model: a comparison between sciatic functional index and kinematic analysis. PLoS ONE. 2018;13:e0208985.
Monte-Raso VV, Barbieri CH, Mazzer N, Yamasita AC, Barbieri G. Is the Sciatic Functional Index always reliable and reproducible? J Neurosci Methods. 2008;170:255–61.
Munro CA, Szalai JP, Mackinnon SE, Midha R. Lack of association between outcome measures of nerve regeneration. Muscle Nerve. 1998;21:1095–7.
Fernandes M, Valente SG, Sabongi RG, Gomes Dos Santos JB, Leite VM, Ulrich H, Nery AA, da Silva Fernandes MJ. Bone marrow-derived mesenchymal stem cells versus adipose-derived mesenchymal stem cells for peripheral nerve regeneration. Neural Regen Res. 2018;13:100–4.
Singh A, Raghav A, Shiekh PA, Kumar A. Transplantation of engineered exosomes derived from bone marrow mesenchymal stromal cells ameliorate diabetic peripheral neuropathy under electrical stimulation. Bioact Mater. 2021;6:2231–49.
Hei WH, Byun SH, Kim JS, Kim S, Seo YK, Park JC, Kim SM, Jahng JW, Lee JH. Effects of electromagnetic field (PEMF) exposure at different frequency and duration on the peripheral nerve regeneration: in vitro and in vivo study. Int J Neurosci. 2016;126:739–48.
Liang L, Liu C, Cai P, Han S, Zhang R, Ren N, Wang J, Yu J, Shang S, Zhou W, et al. Highly specific differentiation of MSCs into neurons directed by local electrical stimuli triggered wirelessly by electromagnetic induction nanogenerator. Nano Energy. 2022;100:107483.
Safavi AS, Sendera A, Haghighipour N, Banas-Zabczyk A. The role of low-frequency electromagnetic fields on mesenchymal stem cells differentiation: a systematic review. Tissue Eng Regen Med. 2022;19:1147–60.
De Pedro J, Pérez-Caballer A, Dominguez J, Collia F, Blanco J, Salvado M. Pulsed electromagnetic fields induce peripheral nerve regeneration and endplate enzymatic changes. Bioelectromagn: J Bioelectromagn Soc Soc Phys Regulat Biol Med Eur Bioelectromagn Associat. 2005;26:20–7.
Mohammadi R, Faraji D, Alemi H, Mokarizadeh A. Pulsed electromagnetic fields accelerate functional recovery of transected sciatic nerve bridged by chitosan conduit: an animal model study. Int J Surg. 2014;12:1278–85.
de Groot MW, Kock MD, Westerink RH. Assessment of the neurotoxic potential of exposure to 50Hz extremely low frequency electromagnetic fields (ELF-EMF) in naive and chemically stressed PC12 cells. Neurotoxicology. 2014;44:358–64.
Baptista AF, Goes BT, Menezes D, Gomes FCA, Zugaib J, Stipursky J, Gomes JR, Oliveira JT, Vannier-Santos MA, Martinez AMB. PEMF fails to enhance nerve regeneration after sciatic nerve crush lesion. J Peripher Nerv Syst. 2009;14:285–93.
Bertagna F, Lewis R, Silva SRP, McFadden J, Jeevaratnam K. Effects of electromagnetic fields on neuronal ion channels: a systematic review. Ann N Y Acad Sci. 2021;1499:82–103.
Kang KS, Hong JM, Kang JA, Rhie JW, Jeong YH, Cho DW. Regulation of osteogenic differentiation of human adipose-derived stem cells by controlling electromagnetic field conditions. Exp Mol Med. 2013;45:e6.
Zha K, Yang Y, Tian G, Sun Z, Yang Z, Li X, Sui X, Liu S, Zhao J, Guo Q. Nerve growth factor (NGF) and NGF receptors in mesenchymal stem/stromal cells: Impact on potential therapies. Stem Cells Transl Med. 2021;10:1008–20.
Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413.
Rafii MS, Tuszynski MH, Thomas RG, Barba D, Brewer JB, Rissman RA, Siffert J, Aisen PS, Team ANS. Adeno-associated viral vector (Serotype 2)-nerve growth factor for patients with alzheimer disease: a randomized clinical trial. JAMA Neurol. 2018;75:834–41.
Wu Q, Xiang Z, Ying Y, Huang Z, Tu Y, Chen M, Ye J, Dou H, Sheng S, Li X, et al. Nerve growth factor (NGF) with hypoxia response elements loaded by adeno-associated virus (AAV) combined with neural stem cells improve the spinal cord injury recovery. Cell Death Discov. 2021;7:301.
Zhang Y, Ding J, Duan W. A study of the effects of flux density and frequency of pulsed electromagnetic field on neurite outgrowth in PC12 cells. J Biol Phys. 2006;32:1–9.
Longo FM, Yang T, Hamilton S, Hyde JF, Walker J, Jennes L, Stach R, Sisken BF. Electromagnetic fields influence NGF activity and levels following sciatic nerve transection. J Neurosci Res. 1999;55:230–7.
Acknowledgements
Sang-Yoon Lee and Bongju Kim contributed equally as a first authors. This research was supported by a grant from the Korea Health Technology R&D Project through Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant number: HI20C2114).
Funding
This work was supported by a grant from the Korea Health Technology R&D Project through Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant number: HI20C2114).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by S-L, BK and S-HL. The first draft of the manuscript was written by KP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All experimental procedures were conducted in compliance with the the care guidelines of the laboratory of animal resources of Seoul National University and approved by the Institutional Animal Care and Use Committee of Seoul National University (SNU-130201-2).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, SY., Kim, B., Lee, SH. et al. Biomechanical microenvironmental stimulating effect of pulsed electromagnetic field on the regeneration of crush injured rat sciatic nerve. Biomed. Eng. Lett. 13, 235–243 (2023). https://doi.org/10.1007/s13534-023-00276-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-023-00276-w