Abstract
Object
Acmella caulirhiza is a medicinal plant traditionally widely used in Cameroon for the management of several pathologies, hence the need for confirming its pharmacological properties. The objective of this study was to evaluate the subacute toxicity of the aqueous extract of leaves and flowers of A. caulirhiza (AE-AC) on Wistar rats.
Methods
Three groups of female rats received the aqueous extract of A. caulirhiza (AE-AC) at 100, 250, or 500 mg kg−1 Bw doses respectively while a normal group (NG) received distilled water by oral intubation at 10 mL kg−1 Bw daily for 28 days. Animals were weighed every 4 days, death and general toxicity signs were recorded. At the end, rats were fasted for 12 h and after diazepam and ketamine anaesthesia, they were sacrificed; blood was collected for blood count and biochemical analysis. The liver integrity was assessed through transaminase activities, total protein, total cholesterol, and glucose levels, and the kidney integrity through the evaluation of uric acid, and electrolytes level. Histology of some vital organs was also carried out.
Results
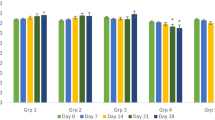
Administration of the extract did not result in death or any observable deleterious effects in rats. No difference in body weight variation of the animals was noted. At 100 and 250 mg/kg Bw doses, AE-AC induced hepatic (through the decrease in transaminase activities and total cholesterol level) and nephroprotective effects (through the decrease in creatinine, uric acid and electrolyte levels) and no change of microarchitecture among treated rats compared to the control group. AE-AC led to an increase in the relative weight of the brain, uterus, and ovaries as well as a change in some haematological parameters compared to normal rats.
Conclusion
Results indicate that AE-AC had immune-stimulatory effects on rats but could have deleterious effects at 500 mg/kg Bw.




Similar content being viewed by others
Data availability
The data employed to support the conclusions of the study are available upon request to the corresponding author.
Abbreviations
- AE-AC:
-
The aqueous extract of Acmella caulirhiza
- ALAT:
-
Alanine Amino Transferase
- ASAT:
-
Aspartate Amino Transferase
- Hb:
-
Hemoglobin
- Hcte:
-
Hematocrit
- Lymp:
-
Lymphocytes
- MCHC:
-
Mean Corpuscular Hemoglobin Concentration
- MCH:
-
Mean Corpuscular Hemoglobin
- MCV:
-
Mean corpuscular volume
- Mon:
-
Monocytes
- NIH:
-
National Institutes of Health
- OECD:
-
Organization for Economic Cooperation and Development
- PCV:
-
Packed cell volume
- PLT:
-
Platelet
- RBC:
-
Red blood cell
- WBC:
-
White blood cells
References
Wang JY, Yuan Y, Chen X, Fu S, Zhang L, Hong Y, You S, Yang Y (2016) Extract from Eucommia ulmoides Oliv. ameliorates arthritis via regulation of inflammation, synoviocyte proliferation and osteoclastogenesis in vitro and in vivo. J Ethnopharmacol 194:609–616. https://doi.org/10.1016/j.jep.2016.10.038
World Health Organization (2008) Traditional medicine (2008) Fact Sheet 134: 2003–2005. Archived from the Original on 28.07.08
Kabbaoui M, Chda A, Azdad O, Mejrhit N, Aarab L, Bencheikh R, Tazi A (2016) Evaluation of hypoglycemic and hypolipidemic activities of aqueous extract of Cistus ladaniferus in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 6(12):1044–1049. https://doi.org/10.1016/j.apjtb.2016.09.005
Kognou AL, Tchamgoue A, Tchokouaha LR, Ngima D, Nthenge-Ngumbau P, Tchinda A, Fokou PV et al (2018) Acute and sub-chronic toxicity studies of Dichaetanthera africana (Hook. F.) Jacq. Fel. (Melastomataceae) stem bark ethanol extract. J Appl Pharm Sci 8(06):147–155. https://doi.org/10.7324/JAPS.2018.8619
Masoko P (2017) Phytochemical analysis, antioxidant and antibacterial properties of Spilanthes mauritiana used traditionally in Limpopo Province, South Africa. J Evid Based Compl Altern Med 22(4):936–943. https://doi.org/10.1177/2515690X17746774
Musila MN, Ngai DN, Mbiri JW, Njagi SM, Mbinda WM, Ngugi MP (2017) Acute and subchronic oral toxicity study of methanolic extract of Caesalpinia volkensii (Harms). J Drug Metab Toxicol 8(1):1–8. https://doi.org/10.4172/2157-7609.1000222
Tang R, Tian R, Cai J, Wua J, Shen X, Hu Y (2017) Acute and sub-chronic toxicity of Cajanus cajan leaf extracts. Pharm Biol 55(1):1740–1746. https://doi.org/10.1080/13880209.2017.1309556
Ochoa M, Reyes V, Sánchez A, Guzmán M, Noguera P, Angeles E, Alba-Hurtado F (2014) Subchronic toxicity study in rats of two new ethyl-carbamates with ixodicidal activity. Biomed Res Int. https://doi.org/10.1155/2014/467105
Etame RME, Mouokeu RS, Ngane RAG, Assam Assam JP, Masoohe AM, Tientcheu R, Hopogap ML, Etoa FX (2017) Acute and sub-acute toxicity of Harungana madagascariensis LAM (Hypericaceae) stem bark methanol extract. J Appl Pharm Sci 7(03):160–167. https://doi.org/10.7324/j.JAPS.2017.70326
Bedoor AS, Muhsin SGA, Ausama AJ (2022) Sub-chronic effects of mefenamic acid alone or in combination with diclofenac on the female reproductive system in albino rats. Toxicol Environ Heal Sci 14:319–326. https://doi.org/10.1007/s13530-022-00145-6
Youovop FJA, Takuissu GRN, Ngondi JL, Oben JE (2022) Acute and sub-chronic toxicity studies of aqueous bark extract of Detarium microcarpum guill. and perr in albinos wistar rat. Toxicol Environ Health Sci. https://doi.org/10.1007/s13530-022-00139-4
Yuet K, Darah I, Chen Y, Sreeramanan S, Sasidharan S (2013) Acute and subchronic toxicity study of Euphorbia hirta L, methanol extract in rats. Biomed Res Int. https://doi.org/10.1155/2013/182064
Kidane B, Tinde A, Laurentis JGM, Zemde A (2014) Use and management of traditional medicinal plants by Maale and Ari ethnic communities in southern Ethiopia. J Ethnobiol Ethnomed. https://doi.org/10.1186/1746-4269-10-46
Yimta F, Mahiou-Leddet V, Nguimatsia F, Mbenkum T, Wouessidjewe D, Evelyne O, et al. (2017) Ethnobotanical survey of medecinal plants used in the western region of cameroon for the treatment of various infectious diseases
Ndjouondo GP, Ngene JP, Ngoule CC, Kidik MC, Ndjib RC, Dibong SD, Mpondo E (2015) Inventaire et caractérisation des plantes médicinales des sous bassins versants Kambo et Longmayagui (Douala, Cameroun). J Animal Plant Sci 25(3):3898–3916
Tesfaye S, Belete A, Engidawork E, Gedif T, Asres K (2020) Ethnobotanical study of medicinal plants used by traditional healers to treat cancer-like symptoms in eleven districts Ethiopia. Evid-Based Complement Altern Med 2020:7683450. https://doi.org/10.1155/2020/7683450
Arora S, Vivay S, Kumar D (2011) Phytochemical and antimicrobial studies on the leaves of Spilanthes acmella. J Chem Pharm Res 3(5):145–150
Lagnika L, Amoussa AM, Adjileye RA, Laleye A, Sanni A (2016) Antimicrobial, antioxidant, toxicity and phytochemical assessment of extracts from Acmella uliginosa, a leafy-vegetable consumed in Bénin. West Afr BMC Compl Altern Med 16(34):34–44. https://doi.org/10.1186/s12906-016-1014-3
Bedi P, Jamwal S, Ellali NZ (2017) Antimicrobial activity of Spilanthes acmella and its chemical composition. Saudi J Med Pharm Sci 3(12):1374–1381
Odhiambo O, Abwao I, Githaiga B, Waithaka N (2019) Antimicrobial activity of Acmella caulirhiza on Candida albican and Escherichia coli. J Medi Life Scie 1(2):48–56. https://doi.org/10.21608/jmals.2019.68363
Onyango J, Onyancha J, Onyuka J, Ochora J, Getonto P, Maina C (2017) Phytochemical studies of Acmella caulirhiza and spermacoce princeae used by postpartum mothers in Nyamira county, Kenya. Int J Sci Res Publ 7(8):591–596
Azame TL, Tembe FE, Njinkio NB, Ngoupayo J, Fokunang CN (2020) Phytochemical screening, healing activity and acute toxicity of the sap of the root of Musanga cecropioides and the aqueous extract of the whole plant Acmella caulirhiza in wistar rats. Health Sci Dis 21(1):1–10
Mbong MA, Edoun LF, Ngandi LC, Youvop JA, Orang R, Tienoue HM, Nwang F, Ngondi JL, Oben J (2020) Comparative study of the protective effect of Cola anomala and Coffea arabica against induced toxicity in rats. J Food Res 9(5):1–13. https://doi.org/10.5539/jfr.v9n5p1
Li H, Jiang X, Shen X, Sun Y, Jiang N, Zeng J et al (2021) TMEA, a polyphenol in Sanguisorba officinalis, promotes thrombocytopoiesis by upregulating PI3K/Akt signaling. Front Cell Dev. https://doi.org/10.3389/fcell.2021.708331
Langkilde S, Mandimika T, Schrøder M, Meyer O, Slob W, Peijnenburg A, Poulsen M (2009) A 28-day repeat dose toxicity study of steroidal glycoalkaloids, α-solanine and α-chaconine in the Syrian Golden hamster. Food Chem Toxicol 47(6):1099–1108. https://doi.org/10.1016/j.fct.2009.01.045
Michalowicz J, Duda W (2007) Phenols—sources and toxicity. Polish J Environ Stud 16(3):347–362
Arpita R, Shruti A, Navneeta B (2017) A review on medicinal plants against cancer. J Plant Sci Agric Res 2(1):1–3
Afolabi SO, Akindele AJ, Awodele O, Anunobi C, Adeyemi OO (2012) A 90-day chronic toxicity study of Nigerian herbal preparation DAS-77 in rats. Complement Altern Med 12(79):2–18. https://doi.org/10.1186/1472-6882-12-79
Ijioma SN, Osim EE, Nwankwo AA, Nwosu OC, Umezurike CB, Nwawuba NI (2018) Relative organ weights and histological changes in Wistar rats treated with a South East Nigerian Polyherbal formulation (Ajumbise). Int J Biochem Res Rev 21(3):1–10. https://doi.org/10.9734/IJBCRR/2018/37304
Amresh G, Singh N, Rao C (2008) Toxicological screening of traditional medicine Laghupatha (Cissampelos pareira) in experimental animals. J Ethnopharmacol 116:454–460. https://doi.org/10.1016/j.jep.2007.12.008
Poston JN, Gernsheimer TB (2019) Glucocorticoids promote response to thrombopoietin-receptor agonists in refractory ITP: a case series. Int J Hematol. https://doi.org/10.1007/s12185-019-02638-6
Makar R, Zhukov O, Sahud MA, Kuter D (2013) Thrombopoietin levels in patients with disorders of platelet production: diagnostic potential and utility in predicting response to TPO receptor agonists. Am J Hematol 88(12):1041–1044. https://doi.org/10.1002/ajh.23562
Merkin A, Mesele B, Tsige K (2018) Effect of Combretum molle (Combretaceae) seed extract on hematological and biochemical parameters. Med Plants Res 12(5):55–63. https://doi.org/10.5897/JMPR2017.6523
Serbina NV, Jia T, Hohl TM, Pamer E (2008) Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 26:421–452. https://doi.org/10.1146/annurev.immunol.26.021607.090326
Putri DDP, Maran GG, Kusumastuti Y, Susidarti RA, Meiyanto E, Ikawati M (2022) Acute toxicity evaluation and immunomodulatory potential of hydrodynamic cavitation extract of citrus peels. J Appl Pharm Sci 12(04):136–145. https://doi.org/10.7324/JAPS.2022.120415
Oyedemi SO, Yakubu MT, Afolayan AJ (2010) Effect of aqueous extract of Leonotis eonurus (L)R. Br leaves in male Wistar rats. Hum Exp Toxicol 29:377–384. https://doi.org/10.1177/0960327110363864
Olorunnisola OS, Bradley G, Afolayan AJ (2012) Acute and sub-chronic toxicity studies of methanolic extract of Tulbaghia violacea rhizomes in Wistar rats. Afr J Biotech 11(83):14934–14940. https://doi.org/10.5897/AJB12.1565
Kamarul MA, Azzeme AM (2019) Plant toxins: alkaloids and their toxicities. GSC Biol Pharm Sci 6(2):21–29. https://doi.org/10.30574/gscbps.2019.6.2.0003
Grifths MR, Strobel BW, Hama JR, Cedergreen N (2021) Toxicity and risk of plant produced alkaloids to Daphnia magna. Environ Sci Eur 33(10):2–10. https://doi.org/10.1186/s12302-020-00452-0
Akindele AJ, Adeneye AA, Salau OS, Sofidiya MO, Benebo AS (2014) Dose and time-dependent sub-chronic toxicity study of hydroethanolic leaf extract of Flabellaria paniculata Cav (Malpighiaceae) in rodents. Front Pharmacol 5(78):1–9
Tatefuji T, Yanagihara M, Fukushima S, Hashimoto K (2014) Safety assessment of melinjo (Gnetum gnemon L.) seed extract: acute and subchronic toxicity studies. Food Chem Toxicol 67:230–235. https://doi.org/10.1016/j.fct.2014.02.030
Sharabi K, Tavares CD, Rines AK, Puigserver P (2015) Molecular Pathophysiology of hepatic glucose production. Mol Asp Med 46:21–33. https://doi.org/10.1016/j.mam.2015.09.003
Solter PF (2005) Clinical pathology approaches to hepatic injury. Toxicol Pathol 33:9–16
Ma JK, Ding J, Zhao H, Liu CM (2014) Puerarin attenuates carbon tetrachloride-induced liver oxidative stress and hyperlipidaemia in mouse by JNK/c-Jun/CYP7A1 pathway. Basic Clin Pharmacol Toxicol 115(5):389–395. https://doi.org/10.1111/bcpt.12245
Li S, Pasquin S, Eid H, Le-Champion A, Saleem A et al (2021) Identification of abietic Aacid as a key component responsible for the renal protective action of abies balsamea, a medicinal plant of the Eastern James Bay Cree Pharmacopeia. Metab Clin Exp 116:15460. https://doi.org/10.1016/j.metabol.2020.154602
Lote C (2012) Principles of renal physiology. Springer, New York, pp 1–19
Schrier RW (2008) Blood urea nitrogen and serum creatinine: not married in heart failure. Circ Heart Fail 1:2–5
Christian AG, Kechi EL, Oshie NC, John ADO, Nwakaego EM, Ahunna AG, Nwobodo NN (2017) Haematological and biochemical changes after exposure to Maerua crassifolia ethanol leaf extract in rats. J App Pharm Sci 06:136–140. https://doi.org/10.7324/JAPS.2017.70619
Zhang S, Lu B, Han X, Xu L, Qi Y, Yin L et al (2013) Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem Toxicol 55:60–69. https://doi.org/10.1016/j.fct.2012.12.041
Kaneko JJ, Harvey JW, Bruss M, Clinical biochemistry of domestic animals [Internet]. Amsterdam; London: Academic; 2008 [cité 11 avr 2020]. Disponible sur: http://www.dawsonera.com/depp/reader/protected/external/AbstractView/S9780080568829
Adeyemi OO, Akindele AJ, Nwumeh KI (2010) Acute and subchronic toxicological assessment of Byrsocarpus coccineus Schum. And Thonn. (Con-naraceae) aqueous leaf extract. Int J Appl Res 3:1–11. https://doi.org/10.1055/s-0028-1084024
Siwe G, Enow-Orock G, Amang A, Mezui C, Dongmo A, Tan P (2015) Acute and subacute toxicological assessment of the leaf aqueous extract of Eremomastax speciosa (Acanthaceae) in Wistar. J Adv Med Pharm Sci 4(1):1–13. https://doi.org/10.9734/JAMPS/2015/18361
OECD (2008) Repeated Dose 28 Day Oral Toxicity Study in Rodents: OECD/OCDE 407. Organization for Economic Co-Operation and Development (OECD), Paris, 1–13
Porwal M, Khan NA, Maheswari KK (2017) Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdenia tenacissima leaves in experimental rats. Sci Pharm 85:29. https://doi.org/10.3390/scipharm85030029
Brian SB, John AK, Elkin S, Onno WVA (2000) Procedure of determining Packed Cell Volume by the microhematocrit method. Approved Standard third Edition 20(18): NCCLS document H7-A3 (ISBN 1–56238–413–9)
Smith A, Bruton J (1979) Farbatlas histologischer Färbemethoden. Schattauer-Verlag Stuttgart und New York, New York
Reitman S, Frankel S (1957) Dosage des transaminases sériques. Am J Clin Pathol 28(1):56. https://doi.org/10.1093/ajcp/28.1.56
Bartels H, Böhmer M, Heierli C (1972) Serum creatinine determination without protein precipitation. Int J Clin Chem 37:193–197. https://doi.org/10.1016/0009-8981(72)90432-9
Fossati P, Prencipe L, Berti G (1980) Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem 6(2):227–231. https://doi.org/10.1093/clinchem/26.2.0227
Trinder P (1959) Determination of glucose in blood using glucose oxidase with an alternative acceptor. Ann Clin Biochem 6:24–27. https://doi.org/10.1136/jcp.22.2.158
Lowry OH, Rosbrough NJ, Al F, Randall RJ (1951) Protein determination using Folin Ciocalteu reagent. J Biol Chem 193(1):265–275
Roeschlau P, Bernt E, Gruber A (1974) Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem 12(5):226
Fossati P, Principe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28(10):2070–2080
Acknowledgements
The authors are thankful to the Laboratory of Animal Physiology of the University of Yaounde 1 and the University Hospital of Yaounde 1 for their technical support in histopathology analysis.
Funding
No funding was provided for this study.
Author information
Authors and Affiliations
Contributions
HMFT, MAAM and FRN designed of the study. HMFT, IM and FLEE realized the experimentation. HMFT and JAFY have analyzed and interpreted the results. HMFT and FLEE have written the manuscript. MAAM, FRN, and EJO revised it. All authors have read and agreed to the final draft.
Corresponding author
Ethics declarations
Conflict of interest
Conflict of interest Huiny Miriane Fotso Tienoue, Françoise Raïssa Ntentie, Mary-Ann Angie Mbong, Ferdinand Larvin Ebouel Edoun, Inelle Makamwe, Janvier Aimé Fotso Youovop, and Enyong Julius Oben declare that we have no conflict of interest.
Ethical approval
The study was approved by the Institutional Committee for Animal and Human Bioethics, CRFD-SVSE of the University of Yaoundé I. The animals were treated following the Institute's guidelines of the National Institutional Ethics Committee of Cameroon (Council EEC 86/609), which has adopted all the protocols recommended by the European Union on the safety of animals in scientific research.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tienoue, H.M.F., Ntentie, F.R., Mbong, MA.A. et al. Sub-acute toxicity study of the aqueous extract from leaves and flowers of Acmella caulirhiza on female albino Wistar rats. Toxicol. Environ. Health Sci. 15, 227–237 (2023). https://doi.org/10.1007/s13530-023-00176-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-023-00176-7