Abstract
Objective
Considerable environmental pollution is commonly caused by plastics in marine ecosystems, and as plastics breakdown, many toxic chemicals, including polycarbonate and bisphenol-A, are released, adversely affecting the health of marine organisms. In this regard, the present work was devoted to study the toxic effect of bisphenol A (BPA), an endocrine disruptor, in the freshwater crucian fish Gambusia affinis.
Methods
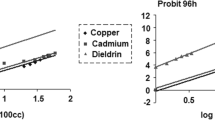
The acute toxicity of BPA was examined in adult male G. affinis with two lethal concentrations, LC25 and LC50, in which the confidence intervals were determined after 24, 48, 72, and 96 h of the exposure period. The oxidative stress markers, including the enzymatic activity of catalase (CAT), glutathione S-transferase (GST), and acetylcholinesterase (AchE), and the contents of malondialdehyde (MDA) and reduced glutathione (GSH) in the hepatopancreas of control and treated fish were determined for 60 days. In addition, the study was included histopathological examinations of testes.
Result
The toxic effect of BPA exposure increased with exposure time and concentration. Chronic exposure to BPA markedly decreased the levels of reduced glutathione (GSH) and the enzymatic activity of brain acetylcholinesterase (AchE), and in contrast, CAT and GST activity and MDA levels were significantly increased.
Conclusions
BPA-exposed fish exhibited severe concentration-dependent acute toxicity, as evidenced by the induction of oxidative injuries, disruption of the neurotransmission process, and subsequently decreased motor activity and alterations in spermatogenesis.








Similar content being viewed by others
References
Caballero-Gallardo K, Olivero-Verbel J, Freeman J (2016) Toxicogenomics to evaluate endocrine disrupting effects of environmental chemicals using the zebrafish model. Curr Genomics 17(6):515–527
Pironti C, Ricciardi M, Proto BPM, Montano L, Motta O (2021) Endocrine-disrupting compounds: an overview on their occurrence in the aquatic environment and human exposure. Water 13(10):1347
Martínez R, Navarro-Martín L, Van Antro M, Fuertes I, Casado M, Barata C, Piña B (2020) Changes in lipid profiles induced by bisphenol A (BPA) in zebrafish eleutheroembryos during the yolk sac absorption stage. Chemosphere 246:125704
United States Environmental Protection Agency (2010). Decontamination research and development conference. U.S. environmental protection agency, Washington, DC, EPA/600/R-11/052, 2011
Schwarzenbach RP, Egli T, Hofstetter Von Gunten U, Wehrli B (2010) Global water pollution and human health. Annu Rev Environ Resour 35(1):109–136
Flint S, Markle T, Thompson S, Wallace E (2012) Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manage 104:19–34
Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, Brooks BW (2015) Global assessment of bisphenol A in the environment: review and analysis of its occurrence and bioaccumulation. Dose-Response 13(3):1559325815598308
Rochester JR (2013) Bisphenol A and human health: a review of the literature. Reprod Toxicol 42:132–155
Faheem M, Bhandari RK (2021) Detrimental effects of bisphenol compounds on physiology and reproduction in fish: a literature review. Environ Toxicol Pharmacol 81:103497
Preethi S, Sandhya K, Lebonah DE, Prasad CV, Sreedevi B, Chandrasekhar K, Kumari JP (2014) Toxicity of bisphenol a on humans: a review. Int Lett Nat Sci 27(2014):32–46
Tohmé M, Prud’homme SM, Boulahtouf A, Samarut E, Brunet F, Bernard L, Laudet V (2014) Estrogen-related receptor γ is an in vivo receptor of bisphenol A. The FASEB J 28(7):3124–3133
Faheem M, Jahan N, Lone KP (2016) Histopathological effects of bisphenol-A on liver, kidneys and gills of Indian major carp Catla catla (Hamilton 1822). J Anim Plant Sci 26(2):514–522
Barboza LGA, Cunha SC, Monteiro C, Fernandes JO, Guilhermino L (2020) Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination. Exposure and risk to human consumers. J Hazard Mater 393:122419
Zhang Q-F, Li Y-W, Liu Z-H, Chen Q-L (2016) Reproductive toxicity of inorganic mercury exposure in adult zebrafish: Histological damage, oxidative stress, and alterations of sex hormone and gene expression in the hypothalamic-pituitary-gonadal axis. Aquat Toxicol 177:417–424
Akram R, Iqbal R, Hussain R, Jabeen F, Ali M (2021) Evaluation of Oxidative stress, antioxidant enzymes and genotoxic potential of bisphenol A in fresh water bighead carp (Aristichthys nobils) fish at low concentrations. Environ Pollut 268:115896
Mukherjee U, Samanta A, Biswas S, Das S, Ghosh S, Mandal DK, Maitra S (2020) Bisphenol A-induced oxidative stress, hepatotoxicity and altered estrogen receptor expression in Labeo bata: impact on metabolic homeostasis and inflammatory response. Ecotoxicol Environ Saf 202:110944
Bustos-Obregon E, Vargas Á (2010) Chronic toxicity bioassay with populations of the crustacean Artemia salina exposed to the organophosphate diazinon. Biol Res 43(3):357–362
Stara A, Pagano M, Capillo G, Fabrello J, Sandova M, Vazzana I (2020) Faggio C (2020a) Assessing the effects of neonicotinoid insecticide on the bivalve mollusc Mytilus galloprovincialis. Sci Total Environ 700:134914
Faheem M, Lone KP (2017) Oxidative stress and histopathologic biomarkers of exposure to bisphenol-A in the freshwater fish Ctenopharyngodon idella. Braz J Pharm Sci. https://doi.org/10.1590/s2175-97902017000317003
Sharma P, Chadha P (2021) Bisphenol A induced toxicity in blood cells of freshwater fish Channa punctatus after acute exposure. Saudi J Biol Sci 28(8):4738–4750
Hamed HS, Abdel-Tawwab M (2017) Ameliorative effect of propolis supplementation on alleviating bisphenol-A toxicity: growth performance, biochemical variables, and oxidative stress biomarkers of Nile tilapia, Oreochromis niloticus (L.) fingerlings. Comp Biochem Physiol C: Toxicol Pharmacol 202:63–69
Maharajan K, Muthulakshmi S, Nataraj B, Ramesh M, Kadirvelu K (2018) Toxicity assessment of pyriproxyfen in vertebrate model zebrafish embryos (Danio rerio): a multi biomarker study. Aquat Toxicol 196:132–145
Epa US (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms; epa-821-r-02-012; us environmental protection agency: washington. DC, USA
Diaz-Sosa VR, Tapia-Salazar M, Wanner J, Cardenas-Chavez DL (2020) Monitoring and ecotoxicity assessment of emerging contaminants in wastewater discharge in the City of Prague (Czech Republic). Water 12(4):1079
Bartoskova M, Dobsikova R, Stancova V et al (2013) Evaluation of ibuprofen toxicity for zebrafish (Danio rerio) targeting on selected biomarkers of oxidative stress. Neuro Endocrinol Lett 34(2):102–108
Abdel-Tawwab M, Hamed HS (2018) Effect of bisphenol A toxicity on growth performance, biochemical variables, and oxidative stress biomarkers of Nile tilapia Oreochromis niloticus (L.). J Appl Ichthyolo 34(5):1117–1125
Fiorino E, Sehonova P, Plhalova L, Blahova J, Svobodova Z, Faggio C (2018) Effects of glyphosate on early life stages: comparison between Cyprinus carpio and Danio rerio. Environ Sci Pollut Res 25(9):8542–8549
Baird SF and Girard CH (1853) Descriptions of new species of fishes collected by Mr. John H. Clark, on the US and Mexican Boundary Survey, under Lt. Col. Jas. D. Graham. In: Proceedings of the Academy of Natural Sciences of Philadelphia.
Bao S, He C, Ku P, Xie M, Lin J, Lu S (2021) Nia X (2021) Effects of triclosan on the RedoximiRs/Sirtuin/Nrf2/ARE signaling pathway in mosquitofish (Gambusia affinis). Aquat Toxicol 230:105679
Song X, Wang X, Li X, Yan X, Liang Y, Huang Y, Huang L (2021) Zeng H (2021) Histopathology and transcriptome reveals the tissue-specific hepatotoxicity and gills injury in mosquitofish (Gambusia affinis) induced by sublethal concentration of triclosan. Ecotoxicol Environ Saf 220:112325
Yazdani M, Andresen AMS, Gjøen T (2016) Short-term effect of bisphenol-a on oxidative stress responses in Atlantic salmon kidney cell line: a transcriptional study. Toxicol Mech Methods 26(4):295–300
Faheem M, Khaliq S, Lone KP (2019) Effect of bisphenol-A on serum biochemistry and liver function in the freshwater fish. Catlacatla Pak Vet J 39(1):1–5
Wu NC, Seebacher F (2020) Effect of the plastic pollutant bisphenol A on the biology of aquatic organisms: a meta-analysis. Glob Change Biol 26(7):3821–3833
Huang GY, Liu YS, Liang YQ, Shi WJ, Hu LX, Tian F, Chen J, Ying GG (2016) Multi-biomarker responses as indication of contaminant effects in Gambusia affinis from impacted rivers by municipal effluents. Sci Total Environ 563:273–281
Afzal G, Ahmad HI, Hussain R, Saeed S, Jamal A, Kiran S, Hussain T, Saeed S, Nisa M (2022) Bisphenol A induces histopathological, hematobiochemical alterations oxidative stress and genotoxicity in common carp (Cyprinus carpio L.). Oxid Med Cell Longev. https://doi.org/10.1155/2022/5450421
Kankaya E, Kaptaner B, Dogan A, Çelik İ (2015) Toxicity of bisphenol a during the early life stages of Chalcalburnus tarichi (Pallas, 1811). Fresenius Environ Bull 24:977–985
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64(2):178–189
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev 2014:360438. https://doi.org/10.1155/2014/360438
Qiu W, Chen J, Li Y, Chen Z, Jiang L, Yang M, Wu M (2016) Oxidative stress and immune disturbance after long-term exposure to bisphenol A in juvenile common carp (Cyprinus carpio). Ecotoxicol Environ Saf 130:93–102
Seoane M, Cid Á, Herrero C, Esperanza M (2021) Comparative acute toxicity of benzophenone derivatives and bisphenol analogues in the Asian clam Corbicula fluminea. Ecotoxicology 30(1):142–153
Zhang J, Shen H, Wang X et al (2004) Effects of chronic exposure of 2, 4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere 55(2):167–174
Aykut H, Kaptaner B (2021) In vitro effects of bisphenol F on antioxidant system indicators in the isolated hepatocytes of rainbow trout (Oncorhyncus mykiss). Mol Biol Rep 48(3):2591–2599
Ali I, Liu B, Farooq MA, Islam F, Azizullah A, Yu SuW, C, Gan, Y, (2016) Toxicological effects of bisphenol A on growth and antioxidant defense system in Oryza sativa as revealed by ultrastructure analysis. Ecotoxicol Environ Saf 124:277–284
Hamed HS, Ali RM, Shaheen AA, Hussein NM (2021) Chitosan nanoparticles alleviated endocrine disruption, oxidative damage, and genotoxicity of Bisphenol-A-intoxicated female African catfish. Comp Biochem Physiol C: Toxicol Pharmacol 248:109104
Kaya Ö, Kaptaner B (2016) Antioxidant defense system parameters in isolated fish hepatocytes exposed to bisphenol A- effect of vitamin C. Acta BiologicaHungarica 67(3):225–235
Uçkun M (2022) Assessing the toxic effects of bisphenol, A in consumed crayfish Astacus leptodactylus using multi biochemical markers. Environ Sci Pollut Res 29(17):25194–25208
Golombieski JI, Marchesan E, Camargo ER, Salbego J, Baumart JS, Loro VL, de Oliveira Machado SL, Zanella R, Baldisserotto B (2008) Acetylcholinesterase enzyme activity in carp brain and muscle after acute exposure to diafuran. Scientia Agricola 65:340–345
Liu H, Yi M, Shi X, Liang P, Gao X (2007) Substrate specificity of brain acetylcholinesterase and its sensitivity to carbamate insecticides in Carassius auratus. Fish Physiol Biochem 33(1):29–34
APHA-AWWA-WEF (1998) In: Clesceri LS, Greenberg AE, Eaton AD (eds) Standard methods for the estimation of water and waste water, 20th edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC.
Buege JA, Aust SD (1984) Microsomal lipid peroxidation. Methods Enzymol 105:302–310
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11(3):151–169
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. Biol Chem 249(22):7130–7139
Bradford M (1976) A rapid and sensitive method for the quantities of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem 72:248–254
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Hould R (1984) Methode de Fontana : Techniques d’histopathologie et de cytopathology. Maloine, Paris
Acknowledgements
This research was supported by the National Fund for Scientific Research of Algeria DGSRTD and by the Ministry of Higher Education and Scientific Research of Algeria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Rahma BELHAMRA, Lazhari TICHATI, Fouzia TREA, Kheireddine OUALI declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants. Animal care was carried out in accordance with Badji Mokhtar-Annaba University animal care protocols.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Belhamra, R., Tichati, L., Trea, F. et al. Effect of subacute treatment with bisphenol A on oxidative stress biomarkers and lipid peroxidation in Gambusia affinis mosquitofish. Toxicol. Environ. Health Sci. 15, 61–72 (2023). https://doi.org/10.1007/s13530-022-00161-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-022-00161-6