Abstract
Objective
Although diabetes patients have a higher propensity to develop infection and sepsis, it is still controversial whether the mortality of sepsis patients is affected by diabetes (DM). We conducted a systematic review and meta-analysis to determine the relationship between diabetes and mortality in patients with sepsis.
Methods
We comprehensively searched for relevant studies in PubMed, MEDLINE, EMBASE, and the Cochrane Library database from January 2000 to December 2021. Two reviewers independently selected studies, extracted data, and assessed quality. We used random-effects modeling to calculate the summary of risk ratios and confidence interval (CI) of mortality. Study quality was assessed using NOS score, and publication bias was assessed using Egger’s statistic.
Results
A total of 23 studies were included in the analyses, comprising 14,521,791 septic patients, including 2,866,429 DM patients. We stratified the in-hospital mortality data by duration for 30 days, 90 day, and mixed days. Meta-analysis of 23 studies showed slightly increased overall mortality among the patients with DM (RR, 1.12; 95% CI 1.00 − 1.25; I2 96.1%; p = 0.000) by pooling of all data in the random effects model. Subgroup analysis did not demonstrate a statistically significant increase either in 30-day mortality (RR, 1.07; 95% CI 0.97–1.18; I2 0.0%; p 0.963), 90-day mortality (RR, 1.00; 95% CI 0.95–1.07; I2 0.0%; p = 0.735), or mixed-day mortality (RR, 1.16; CI 0.98–1.37; I2 97.9%; p = 0.000). The quality of the included studies was good, and the median NOS score was 7.1 (range, 6–9).
Conclusions
This systematic review and meta-analysis of studies suggests that DM does slightly increase sepsis overall mortality, however with statistical heterogeneity. Due to the limitations of the analysis, more well-designed clinical studies are still necessary in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a life-threatening organ dysfunction caused by over activation of inflammatory reaction and coagulation dysfunction response to severe systemic infection. It is a major medical problem worldwide and accounts for 20% of the global death [1]. Diabetes mellitus (DM) is a common and increasing comorbidity in sepsis patients. The incidence rate of DM is rising and has become a major public health problem worldwide [2], especially in low and middle-income countries. Sepsis is closely related to DM; in fact, Sepsis 2.0 used hyperglycemia (blood glucose > 7.7 mmol/L) in patients without a previous history of diabetes as one of the diagnostic criteria for sepsis, which shows the close relationship between sepsis and DM.
It is clear that DM patients are more prone to infection and sepsis, but the impact of diabetes on the outcome of sepsis is still uncertain. Two meta-analyses about this topic showed that presence of diabetes does not increase the risk of mortality in patients with sepsis [3, 4]. Neither of these two meta-analyses included Zoppini’s study [5], a large-size observational study, which proved that diabetic patients had a twofold increased mortality for sepsis compared to non-diabetic patients. Due to the increase of relevant research in recent years, we searched studies January 2000 to December 2021 and conducted a systematic review and meta-analysis on this topic to determine the association between preexisting DM and mortality in humans with sepsis.
Materials and methods
This study protocol was implemented following the Meta-analysis of Preferred Reporting Items for Systematic Reviews (PRISMA) [6].
Data sources and search strategy
We searched PubMed, MEDLINE, EMBASE, and the Cochrane Library database from January 2000 to December 2021. We use medical heading terms and cross search the following three categories for term search: (1) diabetes (“diabetes” or “diabetic”); (2) disease (“sepsis,” “septic shock,” “septic,” or “septicemia”); and (3) others related (“outcome,” “intensive care unit,” “ICU,” “critically ill patients,” “death”, “mortality,” or “prognosis”). We limited the types of studies to “human” and “English” languages. Only studies that reported a comparison between diabetes patients and non-diabetes patients, whose ages were over 18 years of age, were included. All retrieved studies and recent bibliographies were screened to further expand the search scope.
Inclusion criteria
Two researchers independently read the titles and abstracts to determine eligible study. Studies were included if (1) the study population came from a well-established retrospective, prospective cohort, or case–control study, including a group of diabetic patients and a group of non-diabetic patients with sepsis; (2) the 28-day mortality, 90-day mortality, or hospitalization mortality was clearly reported on both group or provided sufficient data to calculate these parameters.
Data extraction and methodological quality
Two researchers (XY and QD) independently collected data from the included studies into a data standardized collection form. The following elements were extracted from the included studies: first author, year of publication, study design, study country, severity of sepsis, and number of diabetes patients and non-diabetic patients. The primary outcome was 28-, 30-, or 90-day mortality and mixed-day mortality. We equated 28-day mortality with 30-day mortality. The day of mortality not specified was assigned to mixed-day mortality. Newcastle–Ottawa Scale tool (available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was used to evaluate the quality of the included studies.
Data synthesis and statistical methods
Stata Software (version 12.0 Stata Corporation, College Station, TX, USA) was used for statistical analysis. The dichotomous data of relative risk (RR) with 95% confidence intervals (CI) for in-hospital mortality in each study were pooled using the random-effects model; results were expressed by Forest plots.
In order to evaluate the effect of DM on the mortality of sepsis patients, we performed a subgroup analysis to evaluate the influence of DM. The first subgroup was sixteen studies that reported the day of mortality not specified. The second subgroup was studies that reported 30-day mortality, and the third subgroup was studies that reported 90-day mortality. Publication bias was assess by Egger’s test [7]. A RR > 1 suggested that DM was associated with an increased risk of mortality.
We proposed to use Cochran’s Q test and reported as I2 to assess and calculate statistical heterogeneity between studies. Sensitivity analysis was used to determine the robustness of the data and the impact of individual research on the summary effect. In addition, p value < 0.05 was considered statistically significant.
Results
Search results
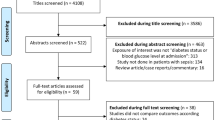
According to the initial search strategy, 5856 unique records were yielded, 1908 duplicates were removed, and 3796 records were eliminated by screening titles and abstracts. Full-text assessment was conducted in the last 156 articles. Of these articles, 23 studies satisfied the inclusion criteria. The study selection process was shown in Fig. 1.
Characteristics of included studies
All the studies were published from 2000 to 2021. There were a total of 14,521,791 septic patients, including 2,866,429 DM patients and 11,646,162 non-DM patients, ages ranging from 45 to 80 years and mostly older than 60 in these studies. Except for 5 prospective studies, the others are retrospective studies. The effect estimations of relative ratios (RRs) of mortality for diabetic patients were provided in each study. What should be mentioned is that four cohorts were included in Russell’s study [8]. Four studies included six cohorts provided relative ratios (RRs) of 30-day mortality for diabetic patients. Four studies provided relative ratios (RRs) of 90-day mortality for diabetic patients. Of these studies, 8 studies enrolled patients with severe sepsis, septic shock patients, or ICU septic patients [8,9,10,11,12,13,14,15], 2 contained non-ICU patients [16, 17], and the left 14 studies enrolled sepsis patients with all stages. The sources of infection in the included studies were not limited to any specific systems or organs. The characteristics of each included study were presented in Table 1.
The quality of each included study, assessed by the Newcastle–Ottawa Scale tool, was good. The NOSs was displayed in Table S1 (median score, 7.1; range from 6 to 9).
Quantitative data synthesis
The mortality RR was estimated using a random-effect model meta-analysis, and heterogeneity was evaluated by I2. Meta-analysis of 23 studies showed that DM did slightly increase sepsis overall mortality (RR, 1.12; 95% CI 1.00–1.25; I2 96.1%; p = 0.000) according to the random effects model, however with large heterogeneity. Subgroup analysis did not demonstrate a statistically significant increase either in 30-day mortality (RR, 1.07; 95% CI 0.97–1.18; I2 0.0%; p = 0.963), 90-day mortality (RR, 1.00; 95% CI 0.95–1.07; I2 0.0%; p = 0.735), or mixed-day mortality (RR, 1.16; 95% CI 0.98–1.37; I2 97.9%; p = 0.000).
Inter-study variability
The pooled relative risk of DM related overall mortality in patients with sepsis was 1.12 (95% CI 1.00–1.25; I2 = 96.1%; p = 0.000). In subgroup analysis, no evidence of heterogeneity was observed in the analysis of 30-day mortality group (I2 = 0.0%; p = 0.963) and 90-day mortality group (I2 = 0.0%; p = 0.735), but a high degree of heterogeneity was observed among mixed-day mortality subgroup (I2 = 97.9%; p = 0.000) and among all the included studies (Fig. 2).
The leave-one-out sensitivity analyses by removing one study per time were used to test the replicability of the results. Two studies [14, 17] were identified as the source of heterogeneity (Fig. 3), and after the exclusion of these two studies, de Miguel-Yanes’ [17] (I2 = 96.7%; p = 0.000) or Shah’s study [14] (I2 = 96.0%; p = 0.000), only a little heterogeneity was removed in the mixed-day mortality subgroup. The omission of de Miguel-Yanes’ [17] or Shah’s study [14] seems to be not drastically changed in this analysis, and the RRs were in the range from 1.13 (95% CI 0.99–1.29) to 1.08 (95% CI 0.98–1.18). All the results were of marginal significance (Fig. 4).
Publication bias
We used Egger’s regression asymmetry test to access the publication bias of included literatures, and no evidence of publication bias could be found (t = 1.64, p = 0.113) (Fig. 4).
Discussion
DM is the main comorbidity of sepsis because of its high prevalence; about 10–30% of septic patients have diabetes. However, the effect of diabetes on outcome of sepsis is not completely clear. There are two meta-analyses about this topic: one showed that the mortality rate of septic patients with DM was slightly lower than that of non-diabetic patients [30]; the other (included four loosely defined sepsis studies) demonstrated that there were no significant differences in the risk of mortality [6]. In a recent meta-analysis, it was reported that DM was associated with mortality, severe COVID-19, ARDS, and disease progression in patients with COVID-19 [30].
In these 23 included studies, the results by Zoppini et al. [7] and Bertoni [18] found increased mortality rate related to sepsis in diabetic compared to the general population, whereas others [9,10,11,12,13, 16, 19,20,21,22,23,24,25,26,27,28,29] failed to demonstrate such association, and four studies [8, 14, 17, 31] reported decreased mortality rates among DM patients during sepsis. The following factors have been proposed to explain this heterogeneity in mortality: different study populations (including different the duration, severity of diabetes, lack of stratification into type 1 and type 2 diabetes, different adjustments for comorbidities, sepsis etiology, stages, and severity) [32], anti-diabetic medication to control blood glucose, degree of glycemic control of during hospital, medical treatment, and nursing. The main finding of our meta-analysis is that pre-existing DM slightly significantly increased overall mortality in sepsis patients, but not 30-day mortality, 90-day, or mixed-day mortality in sepsis patients. From this meta-analysis, it is certain that presence of DM is not associated with reduced risk of mortality in sepsis patients.
To clarify the risk of DM in sepsis mortality, we need to clarify blood glucose level control and the risk of sepsis mortality. As an important cellular energy, blood glucose must be controlled at a specific level and kept relatively stable. Whether it is low or high, it is not conducive to cell survival. It has been demonstrated that hyperglycemia, irrespective of the DM status, is a major independent risk factor for in-hospital sepsis mortality [33], while hypoglycemia is associated with an increased risk of mortality too [15]. Dose–response analysis showed that the effect of blood glucose on mortality may differ in patients with DM versus without [34]. Critically ill patients undergoing intensive glucose control showed significantly reduced all-cause mortality, length of ICU stay, and incidence of acquired infection and sepsis compared to the same parameters in patients treated with the usual care strategy, while the intensive glucose control strategy was associated with higher occurrence of severe hypoglycemic events [35]. Septic patients with higher acute glycemic variability had significantly increased mortality risk compared to those with lower acute glycemic variability; higher acute glycemic variability may be a predictor of mortality risk in patients with sepsis [36]. From these studies, we can draw conclusion that DM should impair the outcome of patients with sepsis; at least, it will not improve the prognosis of sepsis.
In this meta-analysis, the included studies showed a low-risk publication bias. Therefore, the heterogeneity was not considered statistically. The heterogeneity may be derived from methodological and clinical causes, such as the sample sizes, ethnically diverse, anti-diabetic medications, different DM type, different glucose control level, different adjustments for comorbidities, sepsis etiology, and disease severity. The relation between DM and risk mortality is weak across all three subgroups. Due to the weak nature of the association between DM and mortality, drawing conclusions about the practical significance of this relationship should be treated with caution.
This meta-analysis has several strengths. First, the risk publication bias assessment by using Egger’s test showed a low risk of bias among the included studies. All the studies fulfilled the diagnostic criterion proposed by sepsis, and most of the included studies were of high NOS score, which demonstrated the relatively high quality of the included studies. Second, we pooled data for the primary outcome by the random effects model, which allows for more accurate representation of data that arise from complicated multilevel study designs. Finally, the outcome of the sensitivity analysis showed that this result slightly varies.
Study limitations
There are also several limitations in our study which are similar to other meta-analysis. First, there is a marked heterogeneity noted in study design, size, duration, the mean ages, severity, and DM type of the patients among the included studies. Furthermore, most used a retrospective design, and the effect estimate was adjusted for different level confounders. For example, the diagnosis of diabetes in most of the studies depended on the medical history record and did not provide severity, duration, and anti-diabetic medication of diabetics. These heterogeneity might have an effect on the outcome. Second, our analysis only includes the articles published in full text and in English, so the publication bias is unavoidable. Finally, all these limitations of the available data make it hard to reach definitive conclusions of the effect of DM on mortality of sepsis.
Conclusions
Despite diabetes does not increase risk of 28-day mortality or 90-day mortality, it slightly does increase risk of sepsis-related overall mortality. Diabetes is not associated with beneficial survival outcomes in patients with sepsis. Considering the limitations of the meta-analysis, more high-quality original designed studies are required to confirm the association. Future research should aim to gain a deeper understanding of the relationship between DM and mortality using more reliable measures and accurate prospective research to elicit the truth.
Data Availability
The data that support the findings of this study are openly available within the article or its supplementary materials.
Abbreviations
- CI:
-
Confidence interval
- RR:
-
Relative risk
- DM:
-
Diabetes mellitus
References
Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–11.
Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7.
Wang Z, Ren J, Wang G, et al. Association between diabetes mellitus and outcomes of patients with sepsis: a meta-analysis. Med Sci Monit. 2017;23:3546–55.
Jiang L, Cheng M. Impact of diabetes mellitus on outcomes of patients with sepsis: an updated systematic review and meta-analysis. Diabetol Metab Syndr. 2022;14(1):1–17.
Zoppini G, Fedeli U, Schievano E, et al. Mortality from infectious diseases in diabetes. Nutr Metab Cardiovasc Dis. 2018;28:444–50.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(264–269):W64.
Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785–94.
Lin S, Ge S, He W, et al. Association between comorbid diabetes mellitus and prognosis of patients with sepsis in the intensive care unit: a retrospective cohort study. Ann Transl Med. 2021;9(1):22.
Chang CW, Kok VC, Tseng TC, et al. Diabetic patients with severe sepsis admitted to intensive care unit do not fare worse than non-diabetic patients: a nationwide population-based cohort study. PLoS ONE. 2012;7:1–10.
Schuetz P, Kennedy M, Lucas JM, et al. Initial management of septic patients with hyperglycemia in the noncritical care inpatient setting. Am J Med. 2012;125(7):670–8.
Yang Y, Abdul Salam ZH, Ong BC, et al. Respiratory dysfunction in patients with sepsis: protective effect of diabetes mellitus. Am J Crit Care. 2011;20:e41-47.
Moutzouri AG, Athanassiou GA, Dimitropoulou D, et al. Severe sepsis and diabetes mellitus have additive effects on red blood cell deformability. J Infect. 2008;57(2):147–51.
Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–3.
de Miguel-Yanes JM, Mendez-Bailon M, Jimenez-Garcia R, et al. Trends in sepsis incidence and outcomes among people with or without type 2 diabetes mellitus in Spain (2008–2012). Diabetes Res Clin Pract. 2015;110(3):266–75.
Wang J, Zhu CK, Yu JQ, et al. Hypoglycemia and mortality in sepsis patients: a systematic review and meta-analysis. Heart Lung. 2021;50(6):933–40.
Sathananthan M, Sathananthan A, Jeganathan N. Characteristics and outcomes of patients with and without type 2 diabetes mellitus and pulmonary sepsis. J Intensive Care Med. 2020;35:836–43.
Moss M, Guidot DM, Steinberg KP, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28(7):2187–92.
Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care. 2001;24:1044–9.
Zohar Y, ZilbermanItskovich S, Koren S, et al. The association of diabetes and hyperglycemia with sepsis outcomes: a population-based cohort analysis. Intern Emerg Med. 2021;16:719–28.
Akinosoglou K, Kapsokosta G, Mouktaroudi M, et al. Diabetes on sepsis outcomes in non-ICU patients: a cohort study and review of the literature. J Diabetes Complicat. 2021;35(1): 107765.
Kushimoto S, Abe T, Ogura H, et al. Impact of blood glucose abnormalities on outcomes and disease severity in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. PLoS ONE. 2020;15:e0229919.
Russell J, Trinder M, Lee T, et al. Diabetes increases risk of sepsis, but not mortality, inflammatory or lipid responses. SSRN Electron J. 2019. https://doi.org/10.2139/ssrn.3387518.
Van Vught LA, Holman R. De Jonge E et al Diabetes is not associated with increased 90-day mortality risk in critically ill patients with sepsis. Crit Care Med. 2017;45:e1026–35.
Van Vught LA, Scicluna BP, Hoogendijk AJ, et al. Association of diabetes and diabetes treatment with the host response in critically ill sepsis patients. Crit Care. 2016;20:1–15.
Venot M, Weis L, Clec’h C, et al. Acute kidney injury in severe sepsis and septic shock in patients with and without diabetes mellitus: a multicenter study. PLoS One. 2015;10(5):e0127411.
Schuetz P, Jones AE, Howell MD, et al. Diabetes is not associated with increased mortality in emergency department patients with sepsis. Ann Emerg Med. 2011;58(5):438–44.
Stegenga ME, Vincent JL, Vail GM, et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med. 2010;38:539–45.
Vincent J, Preiser J, Sprung C, et al. Insulin-treated diabetes is not associated with increased mortality in critically ill patients. Crit Care Med. 2010;14:1–8.
Chen YC, Jenq CC, Tian YC, et al. classification for predicting in-hospital mortality in critically ill sepsis patients. Shock. 2009;31:139–45.
Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403.
Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care. 2009;13:9–14.
Costantini E, Carlin M, Porta M, et al. Type 2 diabetes mellitus and sepsis: state of the art, certainties and missing evidence. Acta Diabetol. 2021;58(9):1139–51.
Tayek CJ, Tayek JA. Diabetes patients and non-diabetic patient’s intensive care unit and hospital mortality risks associated with sepsis. World J Diabetes. 2012;3(2):29–34.
Wang W, Chen W, Liu Y, et al. Blood glucose levels and mortality in patients with sepsis: dose-response analysis of observational studies. J Intensive Care Med. 2021;36(2):182–90.
Yao RQ, Ren C, Wu GS, et al. Is intensive glucose control bad for critically ill patients? A systematic review and meta-analysis. Int J Biol Sci. 2020;16(9):1658–75.
Li X, Zhang D, Chen Y, et al. Acute glycemic variability and risk of mortality in patients with sepsis: a meta-analysis. Diabetol Metab Syndr. 2022;14(1):59.
Acknowledgements
We would like to thank Xingye Dong for help with figures’ design.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest related to this work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
13410_2023_1225_MOESM1_ESM.docx
Supplementary file1 (DOCX 16 kb) Table S1 The Quality of Studies Assessed by Newcastle–Ottawa Scale. Material S1 This supporting material included: 1.Primary data for Figs. 2 to 4 (XLSX); 2. Command for Figs. 2 to 4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, Q., Yin, X., Zhao, H. et al. Association between comorbid diabetes mellitus and mortality of patients with sepsis: A meta-analysis. Int J Diabetes Dev Ctries 44, 128–136 (2024). https://doi.org/10.1007/s13410-023-01225-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-023-01225-0