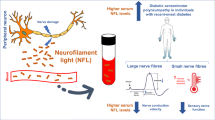
Abstract
Background
Diabetic peripheral neuropathy (DPN) involves a very complex pathogenesis, and there is no neuro-specific marker for risk prediction. Neuron-specific enolase (NSE), a key enzyme in glycolysis, has a broad spectrum neurotrophic and neuroprotective effect on neurons, and also cause injury and inflammatory response of peripheral nerves. The relationship between neuron-specific enolase, highly sensitive c-reactive protein (hsCRP), and diabetic peripheral neuropathy remains unclear. We aimed to investigate whether elevated serum NSE and hsCRP levels increased the risk of DPN in patients with type 2 diabetes.
Materials and methods
In this prospective nested case–control study, a total of 1072 eligible subjects with type 2 diabetes constituted the follow-up cohort. Demographic data and parameters including serum NSE and hsCRP were collected at baseline. Two neuropathy screening scales (MNSI and MDNS) were used to assess DPN during follow-up period. Nerve conduction studies were performed at the end of follow-up. Conditional logistic regression was used to inspect the risk factors of the incidence of DPN.
Results
During an average follow-up period of 5.1 years, 176 subjects developed DPN. Serum NSE and hsCRP levels at baseline were significantly higher in DPN group than in matched non-DPN groups (p < 0.001). NSE was positively correlated with age and hsCRP (p < 0.001). The amplitude of sensory nerve action potential and compound muscle action potential of the lower extremity nerves were significantly decreased in the high tertile of NSE. After adjustment for matching and confusing factor, conditional logistic regression showed the risk of DPN in the high tertile of NSE level was still 3.176 times higher than that in the low tertile of NSE level (p < 0.001).
Conclusion
Elevated serum NSE levels predicted the high incidence of DPN in Chinese patients with type 2 diabetes for an average of 5.1 years, which may be associated with increased neuroinflammatory response caused by high NSE levels, but further studies are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of type 2 diabetes is increasing globally in recent decades. Diabetic peripheral neuropathy (DPN) is one of the main microvascular complications in patients with type 2 diabetes [1]. The prevalence and incidence of DPN in patients with type 2 diabetes varies widely according to the criteria and methods used to define neuropathy [2]. DPN is often ignored by patients due to its insidious onset, large heterogeneity of clinical features, and varying severity of symptoms. DPN is the leading cause of diabetic foot and even amputation, which seriously threatens the health and life safety of patients [3]. Therefore, early prediction and diagnosis of DPN is of great significance for improving the quality of life of patients and reducing the high disability and mortality of DPN.
Neuron-specific enolase (NSE) is a glycolytic enzyme, which mainly exists in neuronal cells, neuroendocrine cells, and related tumor cells [4]. It can promote the survival of neurons in the midbrain and spinal cord under hypoxia, and has a broad spectrum neurotrophic and neuroprotective effect on neurons in central nervous system [5]. Since the pathological characteristics of DPN are neurodegeneration, neuron, and nerve remyelination, the glucose metabolism process and the level of NSE in nerve tissue may have corresponding dynamic changes [6]. NSE may act as a broad-spectrum neurotrophic factor to participate in and guide axon extension and modification of damaged myelin sheaths [7]. In addition, NSE may also be involved in the activation of inflammatory cytokines, chemokines, and other inflammatory mediators, resulting in pathological damage of peripheral nerve tissue [8]. As a commonly used inflammatory marker in clinical practice, high sensitivity C reactive protein (hsCRP) can conveniently provide valuable information about the inflammatory state [9]. By monitoring the expression of NSE and hsCRP, it is possible to analyze the relationship between NSE and inflammatory response in peripheral nerve injury.
In a cross-sectional study, serum NSE levels were higher in patients with diabetes and diabetic neuropathy than in healthy individuals, regardless of blood glucose levels [10]. In another study, serum NSE mRNA expression levels in patients with diabetic neuropathy were significantly lower than those in DM patients and normal individuals [11]. These studies on the correlation between NSE and DPN are cross-sectional studies, with conflicting results and unclear causality.
The onset of DPN involves a variety of abnormal pathophysiological processes. In the pathogenesis of type 2 diabetes, it is not clear whether different expression levels of NES and hsCRP have a promoting effect on the incidence of DPN. The purpose of this study is to explore the potential value of serum NSE and hsCRP in predicting DPN in patients with type 2 diabetes.
Materials and methods
Study design and participant
This was a prospective nested case–control study in China. A total of 1868 individuals with type 2 diabetes were recruited in the diabetes treatment centers of three hospitals affiliated to the PLA Rocket Force Characteristic Medical Center from January 1, 2013 to December 1, 2015. Type 2 diabetes was diagnosed based on the 1999 diagnostic criteria for diabetes of the World Health Organization [12]. Individuals (n = 796) presenting with special clinical diseases at baseline were excluded, including diagnosed diabetic neuropathy (n = 663); peripheral neuropathy other than diabetic origin (n = 102); psychiatric disorders (n = 11); pregnant women (n = 9); malignant tumor (n = 8); and drugs and toxins related neuropathy (n = 3). A total of 1072 eligible subjects with type 2 diabetes constituted the follow-up cohort. The participants’ flow chart is shown in Fig. 1.
Data collection
Demographic and physical examination data at baseline were obtained from the subjects. The items included gender, age, height, weight, smoking history, duration of diabetes, blood pressure, and diagnosed diabetic retinopathy. Body mass index (BMI) was calculated as height divided by the square of weight (kg/m2). The blood pressure, including systolic blood pressure (SBP) and diastolic blood pressure (DBP), was measured three times and the average value was taken. Diabetic retinopathy diagnosed at baseline was assessed by a specialist ophthalmologist using fundus microscopy based on the presence of hard or soft exudates, microaneurysms, bleeding, and new blood vessels in the retina [13].
The data from the laboratory measurement at baseline were collected and evaluated. Blood and urine samples in the morning were collected after the subjects fasted for at least 8 h. The blood samples were centrifuged at 3500 rpm at 4 °C for 10 min and stored at 80 °C until analysis. The fasting serum NSE concentration was determined by electrochemiluminescence immunoassay. Measuring interval was < 16.3 ng/mL (95%); 15.7–17.0 ng/mL (95% confidence interval). Plasma samples were measured in duplicates with intra-assay CV of 2.06% and inter-assay CV of 5.00% for NSE. The glycated hemoglobin was determined by high-performance liquid chromatography, and the value is a percentage (HLC-723G7, Tosoh Corporation, Japan). Levels of total cholesterol (TC), triacylglycerol (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum creatinine (Scr), and fasting plasma glucose (FPG) were measured using an automatic biochemical analyzer (Hitachi 7600 chemical analyzer). C-peptide levels were measured using the chemiluminescence method with an ADVIA Centaur XP automatic analyzer (Siemens Healthcare Diagnostics). Urine albumin concentration was measured by immunoturbidimetric method, urine creatinine concentration was measured by alkaline picric acid, and urine albumin-creatinine ratio (ACR) was calculated by albumin (mg)/creatinine (g). HsCRP were analyzed by enhanced immunoturbidimetric method. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (https://www.niddk.nih.gov).
During follow-up period, patients were called to diabetes medical center of the hospital at a scheduled time each year for formal evaluation by trained medical personnel. DPN was regarded as the primary endpoint of the study and defined using the Michigan Neuropathy Screening Scale (MNSI) and the Michigan Diabetic Neuropathy Score Scale (MDNS). MNSI is recommended to assess the presence of peripheral neurological impairment in patients with diabetes in cohort trials [14]. The inspection content consists of sensory and motor dysfunction such as pain, numbness, and muscle weakness, as well as neurological abnormalities detected by ankle reflexes, a 128-Hz tuning fork vibration perception test, and a 10-g nylon rope tactile test [15]. The items in the study were carried out by trained doctors and nurses.
According to the tertiles of serum NSE levels, 20–25 patients were randomly selected from each group. After obtaining the consent of the patients, nerve conduction function was measured at the end of the follow-up. The electromyography was performed with the Danish Keypoint electromyography instrument by two professional doctors. In a quiet environment, the room temperature was 25–28 °C, and the surface temperature of the patient’s limbs was maintained above 32 °C. The detected nerves included bilateral median nerve, ulnar nerve, common peroneal nerve, tibial nerve, superficial peroneal nerve, and sural nerve. Sensory nerve action potential (SNAP) amplitude, latency and sensory nerve conduction velocity (SCV), compound muscle action potential (CMAP) amplitude, latency and motor nerve conduction velocity (MCV) were measured separately.
Statistical analysis
The statistical analyses were performed using the SPSS Statistics software (SPSS Statistics version 25 for Windows; IBM, New York). Case and control patients were determined by 1:1 case–control matching. Matching criteria: same gender, age difference less than 2 years. Continuous variables with normal distribution were expressed as mean ± SD and analyzed using Student’s t-test or ANOVA, respectively. Data for continuous but non-normally distributed variables were expressed as medians and interquartile ranges and analyzed using non-parametric tests. Categorical data were expressed as percentages and analyzed using chi-square tests. Bivariate associations of NSE with clinical variables were analyzed using Spearman rank correlation analyses. Serum NSE concentration was categorized by tertiles, the low tertile as the reference category, trend test for ANOVA was used to observe the linear trend between groups. Only parameters significant in univariate analysis or known confounding factors to DPN at baseline were added sequentially into the multivariable models. Conditional logistic regression was used to calculate odds ratios (ORs) for incident of DPN and 95% confidence interval (CI) for each risk factor. All statistical tests were two-sided with a level of significance being < 0.05.
Results
After an average follow-up period of 5.1 years, among these 1072 subjects, 971 (90.578%) cases completed the study, 92 cases (8.582%) were lost to follow-up because of no contact or refusal, and 9 (0.840%) cases died. A total of 176 participants with type 2 diabetes developed incident of DPN. Characteristics at baseline of DPN patients and non-DPN patients completed follow-up are shown in Supplementary Table 1.
A total of 176 control patients matched to DPN cases were identified according to 1:1 gender and age matching. Patients with DPN had longer duration of type 2 diabetes; higher ACR,hsCRP and NSE [12.790 (11.043–15.030) vs 10.150 (8.875–11.905) ng/L, t = − 8.039, p < 0.001]; and more diagnosed diabetic retinopathy at baseline than the matched non-DPN. There were no significant differences in smoking history, HbA1c, FPG, peptide, SBP, DBP, TC, TG, HDL-C, LDL-C, and eGFR between the two groups (p > 0.05). The baseline characteristics of the two groups were shown in Table 1.
The level of serum NSE at baseline was positively correlated with age and hsCRP (p < 0.001). There was no correlation between NSE and BMI, duration of type 2 diabetes, ACR, HbA1c, FPG, C-peptide, SBP, DBP, TC, TG, HDL-C, LDL-C, eGFR, etc. (p > 0.05) (Table 2).
Serum NSE at baseline was divided into three groups by tertiles level, respectively, as NSE < 10.175 g/L; 10.175 ≤ NSE ≤ 12.805 g/L; and NSE > 12.805 g/L. With the increase of NSE level, the number of DPN cases, hsCRP, duration of type 2 diabetes, and age at baseline also increased; other parameters as HbA1c, FPG, C-peptide, TC, TG, LDL-C, HDL-C, eGFR, and ACR showed no significant differences (Table 3).
The nerve conduction studies data, including SNAP amplitude, latency and SCV, CMAP amplitude, latency and MCV in each groups, were showed in Table 4. With increasing levels of NSE, the CMAP amplitude of common peroneal nerve and tibial nerve decreased significantly, as well as the SNAP amplitude of superficial peroneal nerve and sural nerve (p < 0.05). SCV and MCV were slightly lower than normal, and there was no significant difference among the three groups. No obvious difference was found in the amplitude and latency of CMAP and SNAP in median and ulnar nerves of upper limbs.
In the conditional logistic regression, after adjusting for confounding factors at baseline such as age, gender, BMI, ACR, HbA1c, hsCRP, and diagnosed diabetic retinopathy, serum NSE at baseline (β = 0.045, SE = 0.013, p = 0.001) and duration of type 2 diabetes (β = 0.077, SE = 0.01, p < 0.001) remained significant predictors of incidence of DPN. By incorporating confounding factors into the model successively, the risk of DPN in the group with the high tertile of NSE level was still 3.176 times higher than that in the group with the low tertile of NSE level (p < 0.001) (Tables 5 and 6).
Discussion
This study was a prospective nested case–control study to investigate the potential value of serum NSE and hsCRP in predicting the risk of DPN in patients with type 2 diabetes. During an average 5.1-year follow-up period, the risk of DPN in patients with type 2 diabetes increases with baseline serum NSE levels. After matching and adjusting the main risk factors at baseline, including age, gender, BMI, duration of type 2 diabetes, hsCRP, HbA1c, ACR, and diagnosed diabetic retinopathy, the risk of DPN in the high tertile of NSE level was still 3.176 times higher than that in the low tertile of NSE level (p < 0.001). As far as we know, this is the first report to clarify the potential link between NSE and the development of clinical DPN in patients with type 2 diabetes in a prospective nested case–control study.
Enolase is a key enzyme in the glycolysis process, catalyzing the dehydration of 2-phosphoglycerate to phosphoenolpyruvate. Among the isoenzymes of enolase, a soluble acid protease that belongs exclusively to neuroendocrine tissues and neurons is also called NSE. NSE may have diagnostic and predictive value in differential diagnosis, disease monitoring, curative effect evaluation and recurrence prediction of nervous system, endocrine system diseases and tumors, etc. [16, 17]. Until now, NSE-related research mainly focuses on the mechanism of central nervous system neurons and neuroendocrine tumors, and its role in the pathophysiology of DPN is still unclear. The expression level and type of enolase in peripheral glial cells (Schwann cells, etc.) may change under abnormal glucose metabolism and stress [18]. Typically, Schwann cells only expresses the non-neuronal form of enolase. Long-term abnormal glucose metabolism leads to Wallerian degeneration or demyelination of peripheral neuropathy. The abnormity in Schwann cells metabolic activity may lead to an increase in NSE synthesis to adapt to the increased energy requirements of cell metabolism. Compared to other types of isoenzymes, NSE is more stable and has neurotrophic and neuroprotective effects on damage to axons and Schwann cells. It is beneficial to promote functional recovery after nerve injury. NSE is involved in the activation of glycolysis pathway and the increase of metabolite expression level. At certain physiological levels, it can help to maintain and restore the function of Schwann cells in damaged nerve fibers and has positive significance for the repair of damaged nerves after peripheral nerve injury [19, 20]. In addition, NSE may also cause axon damage by mediating the activation of neurodegeneration pathways and promoting the activation of inflammatory cytokines, chemokines, and other inflammatory mediators [8]. Therefore, the dynamic observation of NSE expression in peripheral blood can be used to infer the injury and repair of peripheral nerves as well as the level of inflammatory response, which can provide clinical reference for the evaluation of metabolism and function of peripheral nerves.
The relationship between NSE and DPN have been investigated in some literatures. In a cross-sectional study conducted by Li et al. [10], it was found that serum NSE levels in diabetic patients were slightly higher than those in normal glycemic patients (9.1 [1.5] vs 8.7 [1.7], p = 0.037).
Diabetic patients with neuropathy had significantly higher serum NSE levels than diabetic patients without neuropathy (10.8 [2.8] vs 9.1 [1.5], p ≤ 0.001). But in the study of Sandhu et al. [11], the NSE mRNA level was significantly higher in diabetic group (no neuropathy or retinopathy) (n = 22) than in healthy subjects (n = 26), and the NSE mRNA level was lower in diabetic neuropathy group (n = 24) than in diabetic control group (no neuropathy or retinopathy) (n = 22). The limitation of the projects was that they were all cross-sectional studies. Some studies included a small number of cases; differences between ethnic groups might affect the final conclusions. Moreover, the conclusions were not consistent in different study populations, which had no practical reference significance for elucidating the causal relationship between NSE and the incidence of DPN.
Our study showed that the baseline serum NSE level of patients in the DPN group was significantly higher than that of the matched non-DPN group [12.790 (11.043–15.030) vs 10.150 (8.875–11.905) ng/L, t = − 8.039, p < 0.001], although NSE in both groups was within the normal range or slightly exceeded the normal value. Older ages at baseline were independently associated with NSE in this study. Studies have shown that the content of NSE in cerebrospinal fluid increases with age, on average by 1% per year [21]. The level of NSE in peripheral blood also increased with age, which may be similar to that of NSE in cerebrospinal fluid. Secondly, we also found no correlation between NSE and HbA1c at baseline; the relationship between hyperglycemia and NSE was controversial. The literature reports that the level of NSE was independent of hyperglycemic metabolic status (fasting glucose, HbA1c, course of disease, and type of diabetes) and other potential confounding factors affecting NSE levels (such as age, sex, and renal status) [10, 11]. However, the mechanism by which blood glucose levels affect NSE remains unclear. High blood glucose levels may cause a manageable increase in the physiological level of NSE; this suggest that NSE may play a more important role in the pathogenesis of DPN. Thirdly, inflammation is one of the important mechanisms of neuropathy. Oxidative stress and inflammation of the nervous system can be induced in the context of persistent hyperglycemia and ischemia or hypoxia, increasing the risk of peripheral neuropathy [22]. At the same time, the expression level of enolase in nerve tissue is upregulated to increase the repair and survival of peripheral nerve tissue. We speculated that the expression level of NSE may fluctuate in different development stages of diabetes; NSE in peripheral nerve tissue may also be controlled, which is related to the functional state of peripheral nerve tissue. In this study, we selected hsCRP as a marker of inflammation. We found a significant correlation between the baseline NSE and hsCRP, which provides evidence for the involvement of NSE in inflammatory response, but the specific mechanism of action remains unclear. Interestingly, no independent association was found between hsCRP and the incidence of DPN. It is currently believed that NSE has a biological half-life of about 24 h in body fluids, and increased serum levels of NSE may trigger the activation of different cellular pathways leading to neuroinflammation. The pro-inflammatory effects of NSE may involve the activation of MMP-9 and NSE-mediated activation of PI3K and MAPK pathways, leading to the release of inflammatory cytokines and chemokines that contribute to the development of neuropathy [23, 24]. In order to clarify the mode and significance of NSE’s involvement in neuropathic inflammatory mechanisms of DPN, it may be necessary to select more appropriate markers of inflammation.
The most significant finding in this study is that elevated serum NSE levels at baseline is a risk factor for DPN, independent of other known risk factors for DPN. Older ages and duration of type 2 diabetes are common risk factors for DPN, which have been confirmed in ours and other studies [25, 26]. HbA1c has a higher predictive value of DPN in patients with type 1 diabetes than in type 2 diabetes, active hypoglycemic therapy has been shown to be effective in delaying the onset of DPN in type 1 diabetes, but the effect on DPN in type 2 diabetes is not obvious, which is related to the complexity pathogenesis of DPN in type 2 diabetes. Modifiable cardiovascular risk factors are associated with the incidence of neuropathy, including elevated TG levels, BMI, and hypertension, etc. [27]. No statistical significance was found about these indexes in our study.
In nerve conduction studies, with the increase of NSE level, the CMAP amplitude of the motor nerve and the SNAP amplitude of the sensory nerve of the lower limbs were decreased significantly (p < 0.05). The peripheral neuropathy in these patients with type 2 diabetes may be dominated by axonal injury in the distal nerves of the lower limbs, mainly small nerve fibers, and the axonal injury in the high tertiles level of NSE is more significant than that in the low NSE tertiles. Due to the high content of NSE in the axons of peripheral nerves, long-term neuropathy leads to Wallerian degeneration, axonal destruction, and NSE leakage increased. SNAP and CMAP amplitude, which reflect the degree of axonal damage, can be significantly reduced [28, 29]. At the same time, the demyelination of nerve tissue was not obvious. Therefore, there was no significant change of latency, SCV, and MCV in sensory and motor nerve detection. For patients with type 2 diabetes, the low tertile expression level of NSE in nerve tissue has neurotrophic and protective effects on diabetic peripheral nerve tissue, and the high tertile expression level is related to abnormal metabolic status, inflammatory response, and the pathological damage of peripheral nerve tissue. Persistent metabolic abnormalities or irreversible damage of Schwann cells in peripheral nerves indicate the possibility of DPN in the future. The study shows that the risk of DPN in the group with the high tertile of NSE level was still 3.176 times higher than that in the group with the low tertile of NSE level (p < 0.001). This provides support for the possibility that NSE becomes a neuro-specific marker to predict DPN in type 2 diabetes.
There are some limitations in this study. Only Chinese patients with type 2 diabetes were included in this study, which may not be fully representative of the general population. Therefore, we should be cautious when extrapolating the conclusions. In addition, due to the limited sample size and the complexity pathogenesis of DPN, confounders and the influencing factors to NSE were not all included in the study. Third, the diagnosis of DPN is mainly based the neuroscale, and only some patients have nerve conduction studies; the relationship between NSE and the severity of DPN was also not discussed. Lastly, the dynamic changes of NSE during the disease were not fully considered; further adjustments should be made in future studies.
Conclusion
This prospective nested case–control study identified a potential association between NSE and the development of clinical DPN in patients with type 2 diabetes. The results indicate that elevated serum NSE levels increased neuroinflammatory responses and had a predictive value for the occurrence of DPN in patients with type 2 diabetes for an average of 5.1 years. NSE may become a neuro-specific marker for DPN and provide a useful reference for the early diagnosis and treatment of DPN.
Data Availability
The data that support the findings of this study are available from the corresponding upon reasonable request.
Abbreviations
- NSE:
-
Neuron-specific enolase
- hsCRP:
-
High sensitivity C-reactive protein
- T2DM:
-
Type 2 diabetes mellitus
- DPN:
-
Diabetic peripheral neuropathy
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TC:
-
Total cholesterol
- TG:
-
Triacylglycerol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- HbA1c:
-
Glycosylated hemoglobin A1c
- FPG:
-
Fasting plasma glucose
- eGFR:
-
Estimated glomerular filtration rate
- ACR:
-
Urine albumin-creatinine ratio
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- MNSI:
-
Michigan Neuropathy Screening Scale
- MDNS:
-
Michigan Diabetic Neuropathy Score Scale
- ORs:
-
Odds ratios
- CI:
-
Confidence interval
References
Boulton AJ. Diabetic neuropathy: classification, measurement and treatment. Curr Opin Endocrinol Diabetes Obes. 2007;14(2):141–5.
Holzer SE, Camerota A, Martens L, et al. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther. 1998;20(1):169–81.
Young MJ, Boulton AJ, Macleod AF, et al. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150–4.
Fukano K, Kimura K. Measurement of enolase activity in cell lysates. Methods Enzymol. 2014;542:115–24.
Marangos PJ, Schmechel DE. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu Rev Neurosci. 1987;10:269–95.
Dyck PJ, Giannini C. Pathologic alterations in the diabetic neuropathies of humans: a review. J Neuropathol Exp Neurol. 1996;55(12):1181–93.
Medori R, Jenich H, Autilio-Gambetti L, et al. Experimental diabetic neuropathy: similar changes of slow axonal transport and axonal size in different animal models. J Neurosci. 1988;8(5):1814–21.
Haque A, Capone M, Matzelle D, et al. Targeting enolase in reducing secondary damage in acute spinal cord injury in rats. Neurochem Res. 2017;42(10):2777–87.
Osei-Bimpong A, Meek JH, Lewis SM. ESR or CRP? A comparison of their clinical utility. Hematology. 2013;12(4):353–7.
Li J, Zhang H, Xie M, et al. NSE, a potential biomarker, is closely connected to diabetic peripheral neuropathy. Diabetes Care. 2013;36(11):3405–10.
Sandhu HS, Butt AN, Powrie J, et al. Measurement of circulating neuron-specific enolase mRNA in diabetes mellitus. Ann N Y Acad Sci. 2008;1137:258–63.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53.
Lachin JM, White NH, Hainsworth DP, et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015;64(2):631–42.
Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2016;40(1):136–54.
Feldman EL, Stevens MJ, Thomas PK, et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–9.
Shimizu A, Suzuki F, Kato K. Characterization of alpha alpha, beta beta, gamma gamma and alpha gamma human enolase isozymes, and preparation of hybrid enolases (alpha gamma, beta gamma and alpha beta) from homodimeric forms. Biochim Biophys Acta. 1983;748(2):278–84.
Piast M, Kustrzeba-Wójcicka I, Matusiewicz M, et al. Molecular evolution of enolase. Acta Biochim Pol. 2005;52(2):507–13.
Funakoshi T. A clinical study in serum levels of neuron-specific enolase (NSE) in diabetes mellitus. Special reference to types of diabetes and neuropathy. Fukuoka igaku zasshi = Hukuoka acta medica. 1987;78(7):414.
Choi SS, Koh WU, Nam JS, et al. Effect of ethyl pyruvate on paclitaxel-induced neuropathic pain in rats. Korean J Pain. 2013;26(2):135–41.
Kawahara M, Kato-Negishi M, Kuroda Y. Pyruvate blocks zinc-induced neurotoxicity in immortalized hypothalamic neurons. Cell Mol Neurobiol. 2002;22(1):87–93.
van Engelen BG, Lamers KJ, Gabreels FJ, et al. Age-related changes of neuron-specific enolase, S-100 protein, and myelin basic protein concentrations in cerebrospinal fluid. Clin Chem. 1992;38(6):813–6.
Hafner A, Glavan G, Obermajer N, et al. Neuroprotective role of γ-enolase in microglia in a mouse model of Alzheimer’s disease is regulated by cathepsin X. Aging Cell. 2013;12(4):604–14.
Polcyn R, Capone M, Hossain A, et al. Neuron specific enolase is a potential target for regulating neuronal cell survival and death: implications in neurodegeneration and regeneration. Neuroimmunol Neuroinflamm. 2017;4:254–7.
Hafner A, Obermajer N, Kos J. γ-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. Biochem J. 2012;443(2):439–50.
Braffett BH, Gubitosi-Klug RA, Albers JW, et al. Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2020;69(5):1000–10.
Liu X, Xu Y, An M, et al. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PLoS ONE. 2019;14(2):e212574.
Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–50.
Wilson JR, Stittsworth JJ, Kadir A, et al. Conduction velocity versus amplitude analysis: evidence for demyelination in diabetic neuropathy. Muscle Nerve. 1998;21(9):1228–30.
de Souza RJ, de Souza A, Nagvekar MD. Nerve conduction studies in diabetics presymptomatic and symptomatic for diabetic polyneuropathy. J Diabetes Complicat. 2015;29(6):811–7.
Acknowledgements
Thanks for the technical help from the Sixth Medical Center of Soochow University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and approved by the ethics committee of this medical center (2012). Each participant signed the informed consent voluntarily.
Conflict of interest
The authors declare no competing interests. Data availability statement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, J., Du, R., Li, Q. et al. The relationship between neuron-specific enolase, high sensitivity C reactive protein, and diabetic peripheral neuropathy in Chinese patients with type 2 diabetes: A prospective nested case–control analysis. Int J Diabetes Dev Ctries 44, 190–199 (2024). https://doi.org/10.1007/s13410-023-01212-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-023-01212-5