Abstract
Background
There is little clinical evidence that exercise improves prediabetic individuals' glycemic status on glycated hemoglobin A1c (Hb1Ac) and homeostatic model assessment (HOMA) indices. The purpose of this study is to investigate how an eight-week high volume of moderate-intensity endurance plus resistance exercise combined with a medium carbohydrate, low fat, calorie-restricted, carbohydrate counting diet (MCCR) affects Hb1Ac and HOMA indices in individuals with prediabetes.
Methods
Twenty-two young obese people (BMI ≥ 28 kg/m2) were divided into two groups: prediabetes intervention group (INT, n = 10) and normoglycemic control group (CON, n = 12). All participants received the MCCR dietary intervention and a high volume of moderate-intensity endurance plus resistance training, 6 days/week, 5 times/day, and 50 min/time, for 8 weeks. Body composition and circumference, serum lipids, fasting blood glucose (FPG), 2-h post-glucose (2 h-PG), fasting insulin (FINS), Hb1Ac, the insulin resistance (HOMA-IR), insulin sensitivity (HOMA-IS) and β-cell function (HOMA-β) indices were assessed.
Results
After exercise and dietary intervention, Hb1Ac, 2 h-PG, and FINS levels were significantly reduced in both the INT and CON groups (p < 0.05 or p < 0.01). HOMA-IR, HOMA-IS, and body fat percent were significantly improved in the INT group (p < 0.05), but HOMA-β was not observed. Additionally, Hb1Ac levels were significantly normalized in the prediabetic individuals, with a reversion rate of 71.43%, while there was no difference in FPG.
Conclusion
The MCCR diet combined with an eight-week high volume of moderate-intensity endurance and resistance training is effective in reversing Hb1Ac and improving insulin sensitivity in young, obese adults with prediabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prediabetes is an early and inevitable stage of type 2 diabetes mellitus (T2DM) that is characterized by intermediate hyperglycemia, insulin resistance, and islet β malfunction [1]. Prediabetes affects 374 million people worldwide, with that number anticipating a rise to 454 million in 2030 and 548 million in 2045 [2]. A large clinical trial in China showed that prediabetic patients had a 93% high risk of acquiring T2DM after 20 years if no treatment was given [3]. T2DM causes a variety of health issues, such as diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, and vascularity. T2DM patients have a nearly 3.46-fold higher medical cost than those without complications, implying a greater financial burden [4]. Therefore, effective preventive strategies for patients with prediabetes are urgently needed to reduce the global public health threat of T2DM.
In terms of lifestyle, exercise, diet, and weight loss have currently been recognized as the "golden" treatments for preventing T2DM [5]. A clinical investigation found that regular exercise and a balanced diet improved impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) in individuals with prediabetes from Malmö, the United States, and China [6]. Another Diabetes Prevention Program study found that individuals with prediabetes who made lifestyle modifications regained to normal IFG and IGT levels and had a 56% lower risk of developing diabetes [7].
The glycated hemoglobin A1c (Hb1Ac) and HOMA indices are used to screen for glycemic status and β-cell function. Hb1Ac is commonly used as a monitoring indicator for diabetes control in clinic and reflects glycemic exposure in the previous 8 ~ 12 weeks [8]. There is a strong relationship between high Hb1Ac level and the risk of developing diabetes-related complications [9]. Sustained Hb1Ac control was associated with significantly lower odds of being diagnosed with diabetes-related complications five years later [10]. Homeostasis model assessment (HOMA) indices, such as insulin resistance (HOMA-IR), insulin sensitivity (HOMA-IS), and β-cell function (HOMA-β), are often used to quantify β-cell function. In young sedentary female overweight college students, 12-week resistance training may change HOMA-IR and HOMA β [11]. Moreover, Rowan found that a 12-week fitness program that included resistance training, high-intensity interval training (HIIT), or continuous moderate-intensity training would reduce Hb1Ac in prediabetes by only 0.5% [12]. The reason might be related with insufficient physical activity [13]. A high quantity of moderate-intensity exercise and diet management over a 6-month period can significantly enhance oral glucose tolerance and prevent development to T2DM in older adults [14]. Whereas, it is not clear whether moderate-intensity endurance and resistance exercise combined with a MCCR diet can improve Hb1Ac levels and HOMA scores in young adults with prediabetes in a shorter period of time.
The purpose of this study was to compare 1) the effects of an eight-week high volume of moderate-intensity endurance plus resistance training combined with the MCCR diet on reversing Hb1Ac, fasting plasma glucose (FPG), and 2-h post-glucose (2 h-PG) levels, and 2) the effects of a short intervention program on HOMA indices in prediabetic subjects.
Materials and methods
Participants
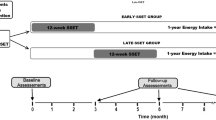
Twenty-two obese adults between the ages of 18 and 30 (body mass indexes, BMI ≥ 28 kg/m2) from the Haoqian Weight loss training camp were divided into the intervention group (obesity and prediabetes; INT, n = 10) and control group (obesity and normoglycemia; CON, n = 12) based on the American Diabetes Association (ADA) prediabetes criteria [15]. All participants were familiarized with the testing procedures and completion of the entire experimental training sessions. In addition, they did not develop any injuries or illnesses that could have affected their training or test performance during the intervention period. Six participants dropped out of the intervention due to personal reasons (Fig. 1). The INT and CON groups were guided by the following criteria of ADA:
-
1)
INT group: obesity (BMI ≥ 28 kg/m2) and prediabetes (5.6 mol/l ≤ FPG ≤ 6.9 mol/l and/or 7.8 mol/l ≤ 2hPG < 11.1 mol/l and/or 5.7% ≤ Hb1Ac ≤ 6.4%).
-
2)
CON group: obesity ((BMI ≥ 28 kg/m2) and normoglycemia (FPG < 5.6 mol/l and/or 2 h-PG < 7.8 mol/l and/or Hb1Ac < 5.7%) [16] (Table 1).
The characteristics of the subjects are described in Table 2. BMI was significantly different in the INT and CON groups (p < 0.05). The study protocol followed the Chinese government's ethical guidelines prior to screening and recruiting. A clinical registration number in the study was ChiCTR2100050506. The epidemiological studies were conducted according to the Declaration of Helsinki and reviewed by the Ethics Committee of Wuhan Sports University. All individuals provided written informed consent.
Methods
Procedure
All subjects received 50 min/time of continuous exercise 5 times/day, 6 days/week for 8 weeks (30 times/week), including 24 aerobic exercise and 6 resistance exercise sessions. Each exercise was done three times a week. The overall time spent on aerobic and resistance exercises was 1200 min/week and 300 min/week, respectively. The workout schedule is detailed in Table 3. Individual tutoring was offered for these subjects under supervision. The Taisin Heart Rate Detection System (DL897) was used to assess heart rate and a rating of perceived exertion (RPE). Subjects must reach 40% ~ 70% of HRmax and be below the RPE score of 13. To guarantee the individuals' safety, the intensity of the exercise was matched to their physical condition.
Participants in two groups were advised to follow a MCCR diet that included meat, shrimp, grains, legumes, vegetables, and fruits. MCCR diet plans were designed by a registered dietitian according to the American Diabetes Association's Complete Guide to Diabetes [17, 18]. They were instructed to eat three meals and two snacks (fruit and milk) per day, to count carbs using 20 g as a unit, and to maintain adequate protein levels. In addition, all participants were advised to consume approximately 220 g of carbohydrates per day and were not permitted to consume alcohol or sugary beverages. According to the Institute of Medicine Dietary Reference Guidelines, the macronutrient dietary composition of the MCCR diet was as follows: 40% ~ 50% from total energy of carbohydrates, 20% ~ 25% from total energy of protein, and < 30% of total energy from fat [19].
Body composition and circumference
All parameters were measured before and after the 8-week intervention during the clinical investigation days. Participants were required to take off shoes, socks, and coats and stand barefoot in the metal area of the X-Scan PLUS II body composition analyzer (JA-WON, Korea) for measuring body composition when they were in a fasting state. Non-stretching tape was used to measure the waist circumference, biceps brachii circumference, and thigh circumference of the participants while they were standing. The measuring tape is placed horizontally on the left side of the body at the hump point of the muscles biceps and left quadriceps to measure the biceps and thigh circumference.
Biomarkers
Blood samples were collected before 10 a.m. and refrigerated at -80 °C. An automated biochemical analyzer was used to measure total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) levels in the blood (Beckman Type Dx800). The glucose oxidase technique was used to assess FPG and 2hPG (the test kit was provided by Tianjin Xiehe Medical Equipment & Supply Co., Ltd., China). A radio-immuno test was used to assess FINS was tested using a radio-immuno test kit given by China's Nanjing Jiancheng Institute of Bioengineering. Hb1Ac was measured using high-performance liquid chromatography (Huadong Electronic DG5033 marker, Nanjing East China Electronics Group Medical Equipment Co., Ltd., China). Homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated according to the formula: HOMA-IR = fasting insulin (µIU/ml) × fasting plasma glucose (mmol/L)/22.5 [20]. The insulin sensitivity index (HOMA-IS) was calculated using the following formula: HOMA-IS = 1/fasting insulin (µIU/ml) × fasting plasma glucose (mmol/L) [21]. Homeostasis model assessment β cell function index (HOMA-β) was calculated according to the formula: 20 × fasting insulin (µIU/ml) / [fasting plasma glucose (mmol/L) − 3.5] [20]. Non-HDL-cholesterol was calculated as total cholesterol minus HDL-cholesterol [22].
Statistical analysis
Experimental data were expressed as meaning ± Standard deviation (M ± SD) and analyzed by SPSS Statistics Version 25.0. The rate of change (%) = intervention data (before—after)/ before × 100%. The two-tailed paired Student's t test was used to determine difference among within group. The independent Student’s t-test was used for comparison between groups. p < 0.05 was considered statistically significant.
Result
Blood glucose
Table 4 presents baselines and change rates of plasma glucose. IFG was defined as 5.6 mol/l ≤ FPG ≤ 6.9 mol/l, while IGT was defined as 7.8 mol/l ≤ 2 h-PG < 11.1 mol/l. Hb1Ac reflects glycemic exposure over the past 2 ~ 3 months, and an increase of 5.7% ~ 6.4% is considered clinically abnormal [16]. As expected, Hb1Ac and 2 h-PG were much lower in the INT and CON groups after 8 weeks of intervention (vs. baselines, p < 0.01; p < 0.05). But FPG had not significantly difference (vs. baselines, p > 0.05). There were significant differences in Hb1Ac and FPG between the INT and CON groups before intervention (INT vs. CON, p < 0.001; p < 0.05), but no difference after intervention (INT vs. CON, p > 0.05). The data were shown in Table 4.
Insulin, insulin resistance, insulin sensitivity and beta cell function index
Insulin is the only hormone in the body that lowers plasma glucose. The insulin resistance index (HOMA-IR), insulin sensitivity index (HOMA-IS), and β-cell function index (HOMA-β) are all methods to evaluate insulin resistance, insulin sensitivity, and β-cell function. Table 4 presents the baselines and change rates of these indexes. The differences in FINS, HOMA-IR, HOMA-IS, and HOMA-β in the INT group were not statistically significant when compared to the CON group.
However, after 8 weeks of intervention, we observed a decrease in insulin secretion compared with baseline in both of the CON and INT groups (vs. baselines, p < 0.05; p < 0.01). Furthermore, HOMA-IR and HOMA-IS were all improved in the INT group (vs. INT baseline, p < 0.05; p < 0.05). HOMA-β was significantly decreased by intervention in the CON groups (vs. baselines, p < 0.05), but not in INF group (vs. baselines, p = 0.05, Table 4).
Serum lipid
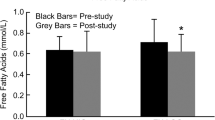
Serum lipids represent the metabolism of fat in the body. Table 4 presents the baselines and change rates of the serum lipid index. In the present study, there were no differences at baseline in TC, TG, LDL-c, HDL-c, and no-HDL-c in the INT groups compared with the CON groups. TC, TG, and no-HDL-c in the INT group were changed considerably after 8 weeks of intervention (vs. INT baseline, p < 0.05; p < 0.05; p < 0.01). Furthermore, intervention significantly reduced TG and No-HDL-c in the CON group (vs. CON baseline, p < 0.05; p < 0.05, Table 4).
Body composition and circumference
Body composition and circumference baselines and rates of change are shown in Table 4. As expected, the intervention significantly reduced all participants’ weight in the INT and CON groups (vs. baselines, p < 0.001; p < 0.001), as well as the BMI (vs. baselines, p < 0.001; p < 0.001), which had a significant difference compared with the CON group before the intervention (INT vs. CON, p < 0.05). It indicated that the intervention reduced the BMI gap between the CON and INT groups. In addition, the CON and INT groups changed significantly in body fat percent (vs. baselines, p < 0.01; p < 0.01), body fat mass (vs. baselines, p < 0.01; p < 0.001) and visceral fat index (vs. baselines, p < 0.01; p < 0.01). Waist hip rate (vs. baselines, p < 0.05; p < 0.01), biceps circumference (vs. baselines, p < 0.001; p < 0.001) and thigh circumference (vs. baselines, p < 0.001; p < 0.001) in both the CON and INT groups were considerably improved in Table 4.
Discussion
The present study explored the effects of a high volume of moderate-intensity endurance plus resistance training combined with the MCCR diet on improving Hb1Ac levels in young people with prediabetes. Hb1Ac is the major predictor of glycemic control [23]. In the present study, 71.43% of individuals with prediabetes restored to normal Hb1Ac levels following an 8-week exercise and dietary intervention.
There are two diagnostic criteria for pre-diabetes from the World Health Organization (WHO) and the ADA [24]. The WHO defines impaired fasting glucose (IFG) as a FPG concentration of 6.1 mol/l (110 mg/dl) ~ 6.9 mol/l (125 mg/dl), and impaired glucose tolerance (IGT) as a 2 h post-load plasma glucose concentration of 7.8 mol/l (140 mg/dl) ~ 11.1 mol/l (199 mg/dl). The ADA's IGT threshold for prediabetes is the same as the WHO's, IFG requires FPG to reach 5.6 mmol/L ~ 6.9 mmol/L (100 mg/dl ~ 125 mg/dl). The ADA has advocated that the diagnostic criterion for Hb1Ac in pre-diabetes is 5.7% ~ 6.4% (39 mmol/mol ~ 46 mmol/mol) [16]. Hence, as compared to WHO standards, the ADA diagnostic criteria for prediabetes are more prone to a "high risk status" and greater prevalence rates of diabetes in the future. In the present study, we used the prediabetes criterion of the ADA for a comprehensive selection of subjects.
The MCCR diet can lower Hb1Ac and establish long-term glycemic management by reducing the postprandial glycemic response. Færch et al. demonstrated that post-prandial glucose uptake was the greatest predictor of Hb1Ac in non-diabetic patients [25]. Increasing exercise-induced glucose uptake in skeletal muscle through a non-insulin-dependent mechanism is beneficial in patients with peripheral insulin resistance [26]. Increased frequency of exercise and calorie restriction contribute to the normalization of Hb1Ac in prediabetes. Although one 5-year follow-up study found that most patients with Hb1Ac—defined prediabetes remained in prediabetes state or progressed to diabetes during the 5-year period, with a reversion rate only 32% [23], the present study indicated that short periods of intervention with intense exercise and a calorie-restriction diet could be effective in reversing Hb1Ac.
According to the present study data, fasting insulin, insulin resistance, and insulin sensitivity in prediabetes improved by 57.42%, 59.80%, and 156.85%, respectively. HOMA-IR is widely used and is significantly correlated with insulin sensitivity in the presence of normal FPG [27]. The population studied in this paper exhibits mean FPG values, suggesting that HOMA can be safely used in these patients, and a high volume of moderate-intensity endurance plus resistance training combined with the MCCR diet is considered an effective protocol for modulating insulin resistance. These results are similar to previous results from the RESOLVE study, which found that 15 ~ 20 h of exercise per week for a total of 3 weeks improved insulin resistance in 11.9% of participants with metabolic syndrome [28]. HOMA-β has limitations when β-cell defects impair the physiological ability to compensate for insulin resistance as insulin increases. The Festa A. et al. demonstrated that HOMA-β underestimated the extent of the β-cell dysfunction in the presence of impaired glucose tolerance [29]. In a prospective UK diabetes study, the β-cell function in patients with T2DM measured by HOMA began to deteriorate 10 ~ 12 years prior to diagnosis, with 50% of β-cell function already lost [30]. When the HOMA-IR score is ≥ 2.5, it can be considered to have insulin resistance [31]. Our findings revealed that the mean HOMA-IR scores in the INF and CON groups of obese participants were 2.8 and 5.1, indicating that they had become insulin resistant. After the sports training and caloric restriction, the HOMA-β values in the CON group decreased, most likely because the interventions improved insulin resistance and compensatory insulin secretion in obese adults. As a result, in obese patients, a lower HOMA-β index does not always indicate impaired β-cell function. More research into the relationship between the HOMA-β index and pancreatic cell function is required. Currently, HOMA-IR and HOMA-IS are still considered the methods of choice for high-risk individuals and are used to differentiate the pathogenesis of T2DM. According to our findings, individuals who engaged in exercise (aerobic up to 20 h/week and resistance up to 5 h/week) and dietary intervention had good effects on insulin resistance and insulin sensitivity in a short period of time.
Interventions that reduce the risk of T2DM by improving body composition and reducing excess visceral fat are considered effective [14]. Calorie restriction has been shown to lower liver fat content in healthy obese individuals. Due to decreased pancreatic and liver triacylglycerol storage, Lim discovered that an 8-week dietary energy restriction alone normalized both β-cell function and hepatic insulin sensitivity in T2DM patients [32]. Obesity was a risk factor for Hb1Ac, which was positively correlated with BMI and WHR [33]. Kahleova et al. also demonstrated that the decrease of insulin resistance was closely related to the loss of visceral fat, and the changes in glucose-induced insulin secretion were related to the change in BMI [34]. In our research, we found that interventions with short-term, extensive exercise and calorie restriction can assist with rapid fat loss in young prediabetes people. Moreover, the sustained weight loss can alleviate the two main pathophysiological changes associated with obese prediabetes: insulin resistance and sensitivity.
According to the National Health and Nutrition Examination Surveys, compared to other risk factors for developing prediabetes, a big waist circumference had the greatest direct effect in prediabetes among adults aged 50 years and older [35]. The benefits of diet control and exercise on physical function and body composition tend to be crucial for clinical interventions to prevent the development of T2DM in high-risk older adults [36]. Furthermore, 12 months of high-intensity progressive resistance training in older patients with T2DM enhanced skeletal muscle mass and lowered Hb1Ac levels [37], and the amount and frequency of exercise had a greater effect on Hb1Ac than the mode or intensity of exercise [38]. Exercise interventions in older adults with prediabetes require a number of considerations due to their declining physical conditions. According to the survey, 28% of older adults aged 53 were prediabetic, with 32% having physical functional limitation and 56% having lower extremity limitation [39]. As a consequence, exercise prescriptions for the elderly may need to be more personalized, emphasizing exercise volume and frequency over exercise mode and intensity. While moderate-intensity exercise is safe, older adults with T2DM should be medically screened before exercising and have their heart rates and blood glucose levels checked throughout prolonged activities to prevent mishaps.
According to our findings, the present prescriptions might be used to lower the occurrence of T2DM in prediabetic individuals by lowering body fat mass and reversing the Hb1Ac level. Significant effects on insulin and blood glucose were detected in the INT and CON groups, respectively, indicating that the short-term intervention program decreased insulin resistance. As a result, these findings have important implications for the selection of clinical intervention to prevent T2DM development in obese individuals at high risk. This is a pilot study of moderate-intensity, high-volume exercise and dietary restriction in young prediabetic patients enrolled in a weight-loss camp. The further studies will investigate the effects of exercise and dietary interventions on indices of prediabetes and explore association between the HOMA-β index and pancreatic cell function in a larger population of older obese adults.
Conclusion
The MCCR diet combined with an eight-week high volume of moderate-intensity endurance and resistance training is effective in reversing Hb1Ac and improving insulin sensitivity in young, obese adults with prediabetes.
Data availability
The data sets for the research presented in the publication are available from the corresponding author on reasonable request.
References
Khetan AK, Rajagopalan S. Prediabetes. Can J Cardiol. 2018;34(5):615–23. https://doi.org/10.1016/j.cjca.2017.12.030.
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. https://doi.org/10.1016/j.diabres.2019.107843.
Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the china da qing diabetes prevention study: A 20-year follow-up study. Lancet. 2008;371(9626):1783–9. https://doi.org/10.1016/S0140-6736(08)60766-7.
Wu H, Eggleston KN, Zhong J, et al. Direct medical cost of diabetes in rural china using electronic insurance claims data and diabetes management data. J Diabetes Investig. 2019;10(2):531–8. https://doi.org/10.1111/jdi.12897.
The diabetes prevention program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–34. https://doi.org/10.2337/diacare.22.4.623.
Eriksson KF, Lindgarde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year malmo feasibility study. Diabetologia. 1991;34(12):891–8. https://doi.org/10.1007/BF00400196.
Perreault L, Pan Q, Mather KJ, et al. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: Results from the diabetes prevention program outcomes study. Lancet. 2012;379(9833):2243–51. https://doi.org/10.1016/S0140-6736(12)60525-X.
Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin a(1c) in the management of diabetes. J Diabetes. 2009;1(1):9–17. https://doi.org/10.1111/j.1753-0407.2009.00009.x.
Mohr DC, Zhang L, Prentice JC, et al. Association of hemoglobin a1c time in range with risk for diabetes complications. BMJ Open Diabetes Res Care. 2022; 10(4). https://doi.org/10.1136/bmjdrc-2021-002738.
Boye KS, Thieu VT, Lage MJ, et al. The association between sustained Hb1Ac control and long-term complications among individuals with type 2 diabetes: A retrospective study. Adv Ther. 2022;39(5):2208–21. https://doi.org/10.1007/s12325-022-02106-4.
Ha CH, Swearingin B, Jeon YK. Relationship of visfatin level to pancreatic endocrine hormone level, homa-ir index, and homa beta-cell index in overweight women who performed hydraulic resistance exercise. J Phys Ther Sci. 2015;27(9):2965–9. https://doi.org/10.1589/jpts.27.2965.
Rowan CP, Riddell MC, Gledhill N, et al. Aerobic exercise training modalities and prediabetes risk reduction. Med Sci Sports Exerc. 2017;49(3):403–12. https://doi.org/10.1249/MSS.0000000000001135.
Richter B, Hemmingsen B, Metzendorf MI, et al. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10:CD012661. https://doi.org/10.1002/14651858.CD012661.pub2.
Slentz CA, Bateman LA, Willis LH, et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: A randomised controlled trial. Diabetologia. 2016;59(10):2088–98. https://doi.org/10.1007/s00125-016-4051-z.
Liu L, Gao B, Wang J, et al. Joint association of body mass index and central obesity with cardiovascular events and all-cause mortality in prediabetic population: A prospective cohort study. Obes Res Clin Pract. 2019;13(5):453–61. https://doi.org/10.1016/j.orcp.2019.08.004.
American Diabetes A. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care. 2021; 44(Suppl 1): S15-S33. https://doi.org/10.2337/dc21-S002.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the american diabetes association (ada) and the european association for the study of diabetes (easd). Diabetes Care. 2012;35(6):1364–79. https://doi.org/10.2337/dc12-0413.
American Diabetes Association Professional Practice C, American Diabetes Association Professional Practice C, Draznin B, et al. 5. Facilitating behavior change and well-being to improve health outcomes: Standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1): S60-S82. https://doi.org/10.2337/dc22-S005.
Trumbo P, Schlicker S, Yates AA, et al. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–30. https://doi.org/10.1016/s0002-8223(02)90346-9.
Adamska A, Polak AM, Krentowska A, et al. Increased serum fetuin-b concentration is associated with homa-beta and indices of liver steatosis in women with polycystic ovary syndrome: A pilot study. Endocr Connect. 2019;8(8):1159–67. https://doi.org/10.1530/EC-19-0243.
He J, Zhang YL, Wang LP, et al. Impact of different stereoisomers of inositol on insulin sensitivity of gestational diabetes mellitus patients. World J Clin Cases. 2021;9(3):565–72. https://doi.org/10.12998/wjcc.v9.i3.565.
Wen J, Huang Y, Lu Y, et al. Associations of non-high-density lipoprotein cholesterol, triglycerides and the total cholesterol/hdl-c ratio with arterial stiffness independent of low-density lipoprotein cholesterol in a chinese population. Hypertens Res. 2019;42(8):1223–30. https://doi.org/10.1038/s41440-019-0251-5.
Vistisen D, Kivimaki M, Perreault L, et al. Reversion from prediabetes to normoglycaemia and risk of cardiovascular disease and mortality: The whitehall ii cohort study. Diabetologia. 2019;62(8):1385–90. https://doi.org/10.1007/s00125-019-4895-0.
Puavilai G, Chanprasertyotin S, Sriphrapradaeng A. Diagnostic criteria for diabetes mellitus and other categories of glucose intolerance: 1997 criteria by the expert committee on the diagnosis and classification of diabetes mellitus (ada), 1998 who consultation criteria, and 1985 who criteria. World health organization. Diabetes Res Clin Pract. 1999;44(1):21–6. https://doi.org/10.1016/s0168-8227(99)00008-x.
Faerch K, Alssema M, Mela DJ, et al. Relative contributions of preprandial and postprandial glucose exposures, glycemic variability, and non-glycemic factors to Hb1Ac in individuals with and without diabetes. Nutr Diabetes. 2018;8(1):38. https://doi.org/10.1038/s41387-018-0047-8.
Evans PL, Mcmillin SL, Weyrauch LA, et al. Regulation of skeletal muscle glucose transport and glucose metabolism by exercise training. Nutrients. 2019; 11(10). https://doi.org/10.3390/nu11102432.
Resnick HE, Bergman RN, Henderson JA, et al. Utility of a surrogate measure of insulin resistance in american indians: the strong heart study. Ethn Dis. 2002;12(4):523–9. http://www.ncbi.nlm.nih.gov/pubmed/12477138. Accessed 24 Nov 2022.
Vinet A, Obert P, Dutheil F, et al. Impact of a lifestyle program on vascular insulin resistance in metabolic syndrome subjects: The resolve study. J Clin Endocrinol Metab. 2015;100(2):442–50. https://doi.org/10.1210/jc.2014-2704.
Festa A, Williams K, Hanley AJ, et al. Beta-cell dysfunction in subjects with impaired glucose tolerance and early type 2 diabetes: Comparison of surrogate markers with first-phase insulin secretion from an intravenous glucose tolerance test. Diabetes. 2008;57(6):1638–44. https://doi.org/10.2337/db07-0954.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (ukpds 33). Uk prospective diabetes study (ukpds) group. Lancet. 1998;352(9131):837–53. http://www.ncbi.nlm.nih.gov/pubmed/9742976. Accessed 24 Nov 2022.
Cho SK, Huh JH, Yoo JS, et al. Homa-estimated insulin resistance as an independent prognostic factor in patients with acute pancreatitis. Sci Rep. 2019;9(1):14894. https://doi.org/10.1038/s41598-019-51466-5.
Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54(10):2506–14. https://doi.org/10.1007/s00125-011-2204-7.
Higuchi R, Iwane T, Suwa K, et al. Adjustment for waist circumference reveals a u-shaped association between glycated hemoglobin levels and body mass index in young adults. Can J Diabetes. 2019;43(3):201–6. https://doi.org/10.1016/j.jcjd.2018.09.007.
Kahleova H, Tura A, Hill M, et al. A plant-based dietary intervention improves beta-cell function and insulin resistance in overweight adults: A 16-week randomized clinical trial. Nutrients. 2018; 10(2). https://doi.org/10.3390/nu10020189.
Bardenheier BH, Bullard KM, Caspersen CJ, et al. A novel use of structural equation models to examine factors associated with prediabetes among adults aged 50 years and older: National health and nutrition examination survey 2001–2006. Diabetes Care. 2013;36(9):2655–62. https://doi.org/10.2337/dc12-2608.
Vieira ER, Cavalcanti F, Civitella F, et al. Effects of exercise and diet on body composition and physical function in older hispanics with type 2 diabetes. Int J Environ Res Public Health. 2021; 18(15). https://doi.org/10.3390/ijerph18158019.
Mavros Y, Kay S, Anderberg KA, et al. Changes in insulin resistance and are related to exercise-mediated changes in body composition in older adults with type 2 diabetes: Interim outcomes from the great2do trial. Diabetes Care. 2013;36(8):2372–9. https://doi.org/10.2337/dc12-2196.
Harmer AR, Elkins MR. Amount and frequency of exercise affect glycaemic control more than exercise mode or intensity. Br J Sports Med. 2015;49(15):1012–4. https://doi.org/10.1136/bjsports-2013-093225.
Lee PG, Cigolle CT, Ha J, et al. Physical function limitations among middle-aged and older adults with prediabetes: One exercise prescription may not fit all. Diabetes Care. 2013;36(10):3076–83. https://doi.org/10.2337/dc13-0412.
Acknowledgments
The authors thank the participants in the Hao Qian weight loss training camp for their contribution in this research.
Funding
This study was funded by the National Natural Science Foundation of China, grant number (81700280, 81970261); the Program of Natural Science Foundation of Hubei Province, grant number 2022CFB478; “Chuyi” High level subject group of rehabilitation therapy technology in Hunan Province.
Author information
Authors and Affiliations
Contributions
Wang M and Zeng SQ developed the study design; Zeng SQ collected data. Zeng SQ, Wang M and Liu H analysis data, edit a draft of the manuscript; Wang M and Liu H review a draft of the manuscript. Liu H, Lou TX, Liu Y, SuP, Feng C, Deng YT and Cheng JW searched literature and investigations; made flow chart; developed the study conception and revised it critically for important intellectual content; Zeng SQ and FC applied for a clinical registration number and Ethics review. All authors read and approved the final manuscript to be published. All the authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
All of the authors say they have no direct or indirect financial or nonfinancial interests in the work that is being submitted for publication.
Ethics approval
The study protocol followed the Chinese government's ethical guidelines prior to screening and recruiting. A clinical registration number in the study was ChiCTR2100050506. The epidemiological studies were conducted according to the Declaration of Helsinki and reviewed by the Ethics Committee of Wuhan Sports University.
Consent to participate
All individuals provided written informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeng, S., Tian, X., Liu, Y. et al. The effect of moderate-intensity endurance plus resistance training combined with MCCR diet on glycemic status in prediabetes. Int J Diabetes Dev Ctries 43, 899–907 (2023). https://doi.org/10.1007/s13410-023-01196-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-023-01196-2