Abstract
Objectives
The prevalence of gestational diabetes mellitus (GDM) has increased year-after-year globally, especially in low-income and developing countries. This study aims to identify the prevalence of GDM, the risk factors, and the effect on pregnancy outcome based on a retrospective case-control study.
Methods
Two hundred ninety-three parturients with GDM who delivered in a general hospital in Fujian province and met the inclusion criteria were selected as the case group from January to June 2018. Two hundred ninety-three parturients without GDM who delivered in the same period served as the control group. Risk factors for GDM were determined by univariate and binary logistic regression analysis. The prevalence of pregnancy outcomes was determined by a chi-square test.
Results
The prevalence of GDM was 15.69%. The percentages of 1, 2, and 3 abnormal OGTT values were 55.6%, 30.7%, and 13.7%, respectively. Gravidas with GDM have a higher risk of macrosomia, polyhydramnios, pre-eclampsia, placenta previa, and gestational hypertension than gravidas without GDM (p < 0.05). Analysis of the factors influencing the development of GDM was advanced age, married, parents with a history of diabetes, gestational hypertension, and number of abortions.
Conclusions
The prevalence of GDM was 15.69% in this geographic region, and > 50% of the patients had one abnormal OGTT value. The risk factors for GDM were advanced age, parents with diabetes, gestational hypertension, and the number of abortions. Pregnancy outcomes of the two groups of patients were different with respect to macrosomia, polyhydramnios, pre-eclampsia, placenta previa, and hypertensive disorders of pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is a decrease in glucose tolerance that occurs or is first detected after pregnancy. The level of blood glucose is lower than that of dominant diabetes [1], which accounts for 80% ~ 90% of gestational hyperglycemia [2]. GDM can cause maternal and infant complications, such as pre-eclampsia, premature rupture of membranes, and premature delivery, and increase the risk of long-term endocrine disorders [3, 4]. Gravidas with GDM have a 70% probability of developing diabetes within 28 years after delivery [5]. The prevalence of GDM is increasing year-after-year globally, especially in low-income and developing countries [6,7,8]. International studies have shown that the risk factors for the occurrence of GDM are not the same in gravidas of different races and residing in different geographic regions [9,10,11]. It is also known that the prevalence and risk factors for GDM are not uniform in different geographic regions of China, and the resulting pregnancy outcomes may also be different [8]. China implemented the “Two-Child Policy” in 2016. A large number of women with advanced maternal age achieved second pregnancies. Lifestyle and dietary imbalance may lead to an increase in the prevalence of GDM. Fujian province has one of the highest GDP rankings in China with a population of 40 million; however, pregnant women in Fujian province with GDM still have a poor sense of how to manage their blood glucose and a deep-rooted belief in eating more and not exercising during pregnancy. Understanding the prevalence and risk factors in the region is conducive to the development of targeted interventions to mitigate the adverse consequences of GDM. This study may determine the prevalence, risk factors, and pregnancy outcomes of parturients with GDM in this geographic region, which is of great significance for researchers to design effective intervention measures further, so as to carry out early diet, exercise, and other interventions for parturients with GDM to improve adverse pregnancy outcomes.
Participants
Participants
This study was reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University in 2019(NO.54). In this study, the convenience sampling method was adopted that maternal inpatients in a general hospital in Fujian province were selected as the study subjects from January to June 2018. The inclusion criteria of the case group were as follows: (1) met the diagnostic criteria for GDM according to the Chinese guidelines for the prevention and treatment of type 2 diabetes [2], (2) single live birth, and (3) no severe pregnancy complications. The exclusion criteria of the case group were as follows: (1) pre-pregnancy diagnosis of diabetes, gestational-dominant diabetes; (2) endocrine diseases, such as hypothyroidism and Cushing’s syndrome; (3) heart, liver, kidney, and other chronic diseases, benign tumors, and multiple pregnancies; and (4) incomplete information. Two hundred ninety-three parturients with GDM were included as the case group, and 293 parturients without GDM hospitalized in the same period were randomly selected as the control group. Inclusion criteria (2 and 3) were the same as the case group, and the exclusion criteria (2–4) were the same as the case group.
Sample
The sample size was based on a maximum likelihood estimation. Thus, the sample size should be > 10 times the number of variables to obtain robust regression analysis results [12]. A total of 17 influencing factors were included in this study, so the minimum sample size for each group was 170 (17 × 10 = 170) and the minimum sample size for two groups was 340. A total of 586 patients were included eventually.
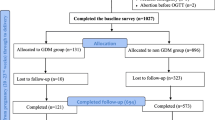
A total of 2666 cases were obtained through the electronic medical record system (EMRS), and 293 cases were included in the case group. A total of 1525 patients in the control group met the standard and were coded according to the sequence of admission numbers and input into an Excel worksheet. The RAND function was used to generate random numbers, with a small-to-large order. The first 293 patients were selected as the control group. A total of 586 patients were included in the two groups (Fig. 1).
Materials and Methods
Instruments
Maternal information questionnaires were used to collect data, including general and disease data, as follows: (1) a self-designed basic information questionnaire was used, including age, marital status, educational level, height before delivery, weight, cigarette smoking, alcohol consumption, family history of diabetes, family history of hypertension, menstrual cycle characteristics, parity, live births and abortions, cesarean section history, and blood pressure (general data); (2) a relevant research group was established to review the literature and group members brainstormed to form the final 21 indicators, including ① neonatal weight, body length, gestational age, preterm birth history, fetal macrosomia, low birth weight, neonatal hypoglycemia, fetal distress, and admission to the NICU; ② oligohydramnios, polyhydramnios, anemia, pre-eclampsia, placental abruption, premature rupture of membranes, placenta previa, hypertensive disorders of pregnancy (HDP), perineal lateral incision, and delivery mode; and ③ the results of the first glucose tolerance test (OGTT) and outliers it contains, and the glycosylated hemoglobin (HbA1c) values examined in the hospital before delivery of the case group (disease data).
Diagnosis of GDM and blood testing
Diabetes can be classified into a single gene diabetes syndrome, post-transplantation diabetes mellitus, cystic fibrosis-related diabetes, prediabetes and type 2 diabetes, type 1 diabetes, and GDM [13]. Gestational hyperglycemia can be divided into pre-pregnancy diabetes mellitus (PGDM), overt gestational diabetes mellitus (OGM), and GDM. GDM accounts for 80% ~ 90% of gravidas with gestational hyperglycemia [2]. In this study, pregnant women with previously undiagnosed diabetes were diagnosed with gestational diabetes using a 75-g glucose tolerance test (OGTT) at 24–28 weeks of gestation. OGTT was performed the morning after a > 8-h overnight fast. GDM was diagnosed when any of the following plasma glucose parameters were reached or exceeded: fasting plasma glucose (FPG) ≥ 5.1 mmol/L but < 7.0 mmol/L, 1-h plasma glucose (1-h PG) ≥ 10.0 mmol/L, and 2-h plasma glucose (2-h PG) ≥ 8.5 mmol/L but < 11.1 mmol/L [1]. After venous blood was obtained from the patient, HbA1c was measured in a laboratory with U.S. Hemoglobin A1c Standardization Program (NGSP) certification and diabetes control and complication test (DCCT) analysis standardized methods. The NGSP uses ion-exchange high-performance liquid chromatography (HPLC) as a reference method and is currently the designated comparison method for HbA1c determination [14].
Data collection
The researcher and two members of the research team created the medical questions regarding the subjects’ past status, retrospectively. They consulted the EMRS in the medical record room to collect the patients’ general and disease data. Before collecting the information, the team members were trained and assessed, including the theoretical knowledge related to GDM, the content of the questionnaire, and the completion specifications. When collecting data, two members worked together, one to consult the electronic medical record and the other to fill in the data form. The collected data were entered into Excel by two members and checked to ensure the accuracy of the data.
Statistical analysis
SPSS 23.0 software was used for statistical analysis. Counting data are shown as frequencies (percentages), and a chi-square test was used for inter-group comparisons. For measurement data conforming to a normal distribution, ‾χ ± S was used to represent the mean value and standard deviation, and t-tests were used for comparison between the two independent samples. Binary logistic regression analysis was used for multivariate analysis. The statistical significance was defined as a p < 0.05.
Results
Prevalence of GDM
Overall prevalence of GDM
The study initially included 2587 patients, including 498 with hyperglycemia in pregnancy and 406 with GDM. GDM accounted for 81.53% of gravidas with hyperglycemia, and the prevalence of GDM was 15.69%.
OGTT of GDM
According to the OGTT results of the case group, 163 patients (55.6%) had one abnormal value, 90 patients (30.7%) had two, and 40 patients (13.7%) had three. Patients with one abnormal value included 56 patients (34.4%) with abnormal FPG, 67 patients (41.1%) with abnormal 1-h plasma glucose, and 40 patients (24.5%) with abnormal 2-h plasma glucose. Fifty-two patients (57.8%) had abnormal FPG and 2-h plasma glucose; 17 patients (18.9%) had abnormal FPG and 1-h plasma glucose, and 21 patients (23.3%) had abnormal 1-h and 2-h plasma glucose. Patients with three abnormal values included 40 patients with an abnormal FPG, 1-h plasma glucose, and 2-h plasma glucose.
Comparison of general information between the two groups
The mean maternal age of the case group was 31.95 ± 5.01 years, while the mean maternal age of the control group was 29.97 ± 4.30 years (Table 1).
Risk factor analysis of GDM
Single-factor analysis
Univariate analysis was performed with GDM as the dependent variable and patient general data as the independent variable. The results showed that age, marital status, paternal diabetes history, maternal diabetes history, parity, the number of pre-pregnancy abortions, and blood pressure were statistically significant(p < 0.05; Table 1).
Multi-factor analysis
Multivariate logistic regression analysis was carried out, including age, marriage, paternal diabetes history, maternal diabetes history, hypertension, the number of pregnancies before the pregnancy, and the number of abortions before the pregnancy. Advanced age, married, paternal diabetes history, maternal diabetes history, HDP, and number of abortions were independent risk factors for GDM. The results are shown in Table 2, according to the size of the standard regression coefficient in order as follows: history of >3 miscarriages, maternal diabetes history, paternal diabetes history, married, a history of two abortions, HDP, a history of one abortion, and age.
Comparison of disease data between the two groups (Table 3)
Discussion
Prevalence of GDM
Prevalence
The prevalence of GDM in this study was 15.69%. A meta-analysis involving the prevalence of GDM in China showed that the prevalence in different geographic regions and hospitals of China varied greatly, ranging from 11.45–23.19% [8]. The prevalence of GDM in the current study was less than the 23.19% prevalence in Beijing [8], which may be related to the sample size, different attributes of research institutions, and patients’ medical habits. There are many general hospitals in Beijing, and high-risk pregnant patients often choose general hospitals for medical treatment. The results of this study are quite consistent with the results of the study on the prevalence of GDM in Fuzhou [8] (14.42%), which may be due to the fact that this region is close to the above area, and the dietary habits, living standards, and lifestyles are similar.
Abnormal blood glucose distribution
Studies have shown that maternal blood glucose levels are associated with the accompanying diabetes status and the prevalence of perinatal complications [15]. In this study, the percentage of abnormal OGTT values in 293 GDM patients was similar to the results of Ding et al. [16]. Saldana et al. [17] reported that as the abnormal OGTT values increased, the risk of complications among gravidas with GDM increased. Moreover, the prevalence of fetal macrosomia with three abnormal OGTT values was the highest [17], which may be due to the fact that two or three outliers may destroy glucose balance and insulin sensitivity more than one outlier, and the higher the number of outliers, the higher the prevalence of adverse pregnancy outcomes [18]. Therefore, abnormal OGTT values have a predictive value for pregnancy outcomes. It has been shown that OGTT fasting hyperglycemia is significantly correlated with macrosomia (OR = 1.84, 95% CI: 1.39 ~ 2.42, p < 0.001), and the correlation is stronger with the increase in FPG [18]. Therefore, stratified management for pregnant women with GDM is recommended, especially for pregnant women with three abnormal OGTT values and a high FPG.
Factors influencing GDM
The results of this study showed that advanced age, married, history of diabetes in parents, HDP, and number of miscarriages were independent risk factors for GDM.
Advanced age
The results of this study showed that the risk of GDM was 2.117 times higher in mothers ≥35 years of age than mothers <35 (OR = 2.117, p < 0.05). This result is consistent with previous studies [6, 19] for the following reasons: ① an increase in age, glucose metabolic differences, lipid metabolic differences, and hormone level changes in females; ② an increase in age, and decline in insulin secretion; and ③ insulin antagonists increased in elderly pregnant women [19]. China implemented the “Universal Two-child Policy” in 2016. The proportion of elderly pregnant women has increased [20]. Therefore, the prevention of GDM in elderly pregnant women is one of the key issues Chinese medical workers are facing, suggesting that we need to focus on exploring the intervention model for pregnant women with advanced maternal age to prevent GDM.
Marriage
In this study, the risk of GDM in married women was 4.393 times higher than unmarried women (OR = 4.393, p < 0.05). The effect of marital status on GDM has not been reported. This finding may reflect the younger age of unmarried parturients in this study than married parturients. There was a significant difference in the married-to-unmarried ratio in this study, which may be related to sample size bias. Therefore, it is not prudent to assume that being married is a risk factor for GDM. In the future, the sample size should be further increased and stratified analysis should be adopted to exclude the influence of age and analyze the influence of marriage on GDM.
History of diabetes in parents
The results of this study showed that patients whose fathers had a history of diabetes had a 4.999 times higher risk of developing GDM than those whose fathers did not have diabetes (OR = 4.999, p < 0.05). Patients whose mothers had a history of diabetes had a 5.088 times greater risk of developing GDM than those whose mothers did not have diabetes(OR = 5.088, p < 0.05), indicating that a history of diabetes in either parent is an independent risk factor for GDM. This finding was consistent with the view that a family history of diabetes is a risk factor for GDM [21] and may be related to the genetic susceptibility of pregnant women with a family history of diabetes, which may induce GDM after pregnancy. The study found that if both parents were diabetic, the prevalence of GDM in the offspring increased [22]. Other studies have shown that a maternal diabetes history has a greater impact on the prevalence of GDM than paternal diabetes, which may be associated with abnormal glucose metabolism in some mothers during pregnancy, leading to fetal dysplasia in utero and further development of GDM [23]. For women of childbearing age who have a history of diabetes in their parents, they should pay more attention to their own blood glucose levels, then prevent, detect, and treat hyperglycemia early.
Hypertension during pregnancy
The study results showed that gravidas with HDP had a 3.598 times higher risk of developing GDM than gravidas without HDP (OR = 3.598, p < 0.05). In agreement with the findings of Abdalrahman [24], the prevalence of GDM in pregnant women with HDP was 2.6 times than gravidas without HDP (OR = 2.6, 95% CI: 1.1~6.2, p = 0.03) for the following reasons: ① insulin resistance, long-term high glucose levels, promotion of fat transformation, feedback imbalance of fat-insulin secretion axis, and change in fat metabolism in pregnant women with GDM; and ② extensive vascular lesions caused by GDM, then vascular endothelial thickening, narrowed lumen, increased vascular resistance, and elevated blood pressure [25]. Therefore, it is necessary to pay attention to blood pressure and blood lipids, as well as blood glucose, in the population with GDM.
Number of miscarriages
The results of this study showed that the risk of GDM was 2.318 times higher in women who had one previous miscarriage than women who did not have a history of miscarriages (OR = 2.318, p < 0.05). Women who had two miscarriages had 4.356 times increased risk of GDM (OR = 4.356, p<0.05). Women who had three or more miscarriages had 6.382 times increased risk of developing GDM (OR = 6.382, p < 0.05). The higher the number of miscarriages, the greater the risk of GDM, which may be related to an increase in the number of pregnancies, the greater the possibility of weight retention-type obesity in pregnant women [26], which may be related to the education level of the pregnant women. It is thought [27] that women who have had multiple abortions are more likely to have poor living habits, a lower social status, and lower educational level and are more likely to have incomplete pre-pregnancy and prenatal health care. Therefore, this group has a greater risk of developing GDM. Attention should be paid to the prevention of GDM for women with a previous history of abortion, especially those who have had three or more abortions.
Effects of GDM on pregnancy outcomes
The results of this study showed that the prevalence of macrosomia, polyhydramnios, pre-eclampsia, placenta previa, and HDP in the case group was statistically different from the control group(p < 0.05). This conclusion is consistent with the research conducted by Kosus et al. [28] and Kamana et al. [29]. GDM can cause short- and long-term maternal, fetal, and newborn complications [1]. Due to the high glucose status of GDM, the secretion of insulin will be stimulated by β cells, then protein synthesis increases, and the risk of macrosomia also increases [3, 4]. The prevalence of macrosomia in the two groups of this study was lower than the above studies, which may be related to the differences in population, research institutions, and dietary habits and lifestyle in different countries. In addition, the recent effects of GDM on maternal and infants include polyhydramnios, pre-eclampsia, HDP, placenta previa, ketoacidosis, premature rupture of membranes, and placental abruption. Gravidas with GDM can reduce the risk of adverse pregnancy outcomes, at least in part, through dietary control, enhanced exercise, weight control, and other interventions [1].
Limitations
Data collection was limited by conditions and time. For example, information on pre-pregnancy BMI, changes in BMI during pregnancy, dietary intake, exercise during pregnancy, and long-term prognosis after childbirth could not be accurately obtained. This study only collected data from one hospital, the sample size was small, and there was a low positive rate for some indicators, which may have affected the stability of the research results based on the limited time and researchers.
Conclusions
In conclusion, the prevalence of GDM in this geographic region was relatively high, and the maternal and infant pregnancy outcomes of the two groups of patients were different with respect to macrosomia, polyhydramnios, pre-eclampsia, placenta previa, and HDP. Advanced age, history of diabetes in the parents, HDP, and number of abortions were independent risk factors for GDM. In view of the above risk factors, the community can strengthen education for high-risk populations, determine effective prevention strategies, construct and improve the management model of early intervention for GDM patients, achieve early detection, treatment, and intervention, strictly control blood glucose levels, and improve maternal and infant outcomes.
References
American Diabetes Association. Management of diabetes in pregnancy: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S137–43.
Yang HX, Song G. Advances in the diagnosis and treatment of gestational diabetes. Chin J Front Med (Electron Ed). 2010;2(3):29–31.
Cheng J. Effect of individualized medical nutrition therapy on blood glucose control efficiency and pregnancy outcome of gestational diabetes mellitus. Shandong: Qingdao University; 2013.
Valkama A, Koivusalo S, Lindstrom J, et al. The effect of dietary counselling on food intakes in pregnant women at risk for gestational diabetes: a secondary analysis of a randomised controlled trial radial. Eur J Clin Nutr. 2016;70(8):912–7.
Alonso A, Del Rey CG, Navarro A, et al. Effects of gestational diabetes mellitus on proteins implicated in insulin signaling in human placenta. GynecolEndocrinol. 2006;22(9):526–35.
Nava GEN, Salcedo GA, Hernández ECE, et al. Prevalence, risk factors and perinatal outcomes of gestational diabetes in Mexican adolescents when applying diagnostic criteria from three different international guidelines. Int J Diabetes Dev Ctries. 2020. https://doi.org/10.1007/s13410-020-00876-7.
Bao W, Tobias DK, Hu FB, et al. Pre-pregnancy potato consumption and risk of gestational diabetes mellitus: prospective cohort study. BMJ. 2016;352:h6898.
Narenqimuge, Li DM, Mi LX, et al. Meta-analysis of prevalence of gestational diabetes in China. Chin J Evid Based Med. 2018;18(3):280–5.
Mohan MA, Chandrakumar A. Evaluation of prevalence and risk factors of gestational diabetes in a tertiary care hospital in Kerala[J]. Diabetes Metab Syndr. 2016;10(2):68–71.
Ganapathy A, Holla R, Darshan BB, Kumar N, Kulkarni V, Unnikrishnan B, et al. Determinants of gestational diabetes mellitus: a hospital-based case–control study in coastal South India. Int J Diabetes Dev Ctries. 2020;41:108–13. https://doi.org/10.1007/s13410-020-00844-1.
Han Y, Tong M, Jin L, Yu J, Meng W, Ren A, et al. Maternal age at pregnancy and risk for gestational diabetes mellitus among Chinese women with singleton pregnancies. Int J Diabetes Dev Ctries. 2020;41:114–20. https://doi.org/10.1007/s13410-020-00859-8.
Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9.
Nathan DM. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes response to Kilpatrick, Bloomgarden, and Zimmet. Diabetes Care. 2009;32(12):e160.
Dong H, Chen WX, Zhang CB, et al. The guideline for glycosylated hemoglobin laboratory. Chin J Diabetes. 2013;21(08):673–8.
Jain R, Davey S, Davey A, Raghav SK, Singh JV. Can the management of blood sugar levels in gestational diabetes mellitus cases be an indicator of maternal and fetal outcomes? The results of a prospective cohort study from India. J Fam Community Med. 2016;23(2):94–9.
Ding TT, Xiang J, Luo BR, Hu J. Relationship between the iadpsg-criteria-defined abnormal glucose values and adverse pregnancy outcomes among women having gestational diabetes mellitus: a retrospective cohort study. Medicine (Baltimore). 2018;97(43):e12920.
Saldana TM, Siega-Riz AM, Adair LS, Savitz DA, Thorp JM. The association between impaired glucose tolerance and birth weight among black and white women in central North Carolina. Diabetes Care. 2003;26(3):656–61.
Feng H, Zhu WW, Yang HX, Wei YM, Wang C, Su RN, et al. Relationship between oral glucose tolerance test characteristics and adverse pregnancy outcomes among women with gestational diabetes mellitus. Chin Med J. 2017;130(9):1012–8.
Yang HL, Yang Z. Effects of advanced gestation on maternal and fetal outcomes. Chin J Emerg Obstetr. 2016;5(03):129–35.
Chen SF, Zhang C, Chen Y, et al. Analysis of factors influencing obstetric safety and countermeasures of reproductive trendon“Two Child Policy”. J Shanghai Jiaotong Univ (Med Ed). 2016;36(5).
Zhang C, Rawal S, Chong YS. Risk factors for gestational diabetes: is prevention possible? Diabetologia. 2016;59(7):1385–90.
Eades CE, Cameron DM, Evans JMM. Prevalence of gestational diabetes mellitus in Europe: a meta-analysis. Diab Res Clin Pract. 2017;129:173–81.
Harder T, Plagemann A. A role for gestational diabetes in the excess maternal transmission of type 2 diabetes? Diabetes Care. 2000;23(3):431–2.
AbdalrahmanAlmarzouki A. Maternal and neonatal outcome of controlled gestational diabetes mellitus versus high risk group without gestational diabetes mellitus: a comparative study. Med Glas (Zenica). 2013;10(1):70–4.
Bardenheier BH, Elixhauser A, Imperatore G, Devlin HM, Kuklina EV, Geiss LS, et al. Variation in prevalence of gestational diabetes mellitus among hospital discharges for obstetric delivery across 23 states in the United States. Diabetes Care. 2013;36(5):1209–14.
Tian YM. Preliminary analysis on prevalence of gestational diabetes mellitus and related risk factors. Shanxi: Shanxi Medical University; 2019.
Wang JF. Risk factors of gestational diabetes mellitus and its effect on pregnancy outcome. Chin J Med. 2017;14(24):135–8.
Kosus N, Kosus A, Duran M, et al. Effect of number of abnormal oral glucose tolerance test (ogtt) values on birthweight in women with gestational diabetes. Indian J Med Res. 2013;137(1):95–101.
Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(2):14–20.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Funding
This research was funded by Quanzhou science and technology plan project in China.
Author information
Authors and Affiliations
Contributions
Inspiration and thinking, guidance: Zhao Huifen
Research design: Zhao Huifen, Xie Yaping, Liu Chunhong, Huang Fengfeng, Zhao Meijing, Huang Huibin
Data collection and statistic analysis: Zhao Huifen, Xie Yaping, Liu Chunhong, Huang Fengfeng, Zhao Meijing, Huang Huibin
Writing manuscript: Zhao Huifen, Xie Yaping, Liu Chunhong
Modify paper and afford guidance: Zhao Huifen, Xie Yaping
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the No.54 file, 2019 of ethics committee of the Second Affiliated Hospital of Fujian Medical University, China.
Consent to participate
This study obtained informed consent from the subjects.
Consent for publication
The article is the authors’ original work. The article has not received prior publication and is not under consideration for publication elsewhere. All authors have seen and approved the manuscript being submitted.
Conflict of interest
All authors report no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Précis Since China implemented the “Two-Child Policy” in 2016, a large number of women with advanced maternal age achieved second pregnancies, which may lead to an increase in the prevalence of GDM.
Quick points
(1) The prevalence of GDM was 15.69% in this geographic region, and > 50% of the patients had 1 abnormal OGTT value.
(2) The risk factors for GDM were advanced age, parents with diabetes, gestational hypertension, and the number of abortions.
(3) The pregnancy outcomes in the two groups of patients differed with respect to macrosomia, polyhydramnios, pre-eclampsia, placenta previa, and hypertensive disorders of pregnancy.
Xie Yaping is the first author. Liu Chunhong is the co-first author.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yaping, X., Chunhong, L., Huifen, Z. et al. Risk factors associated with gestational diabetes mellitus: a retrospective case-control study. Int J Diabetes Dev Ctries 42, 91–100 (2022). https://doi.org/10.1007/s13410-021-00947-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-021-00947-3