Abstract
Background
Observational studies in South Asian populations have suggested an association between vitamin B12 status and metabolic traits; however, the findings have been inconclusive. Hence, the aim of the present study was to use a genetic approach to explore the relationship between metabolic traits and vitamin B12 status in a Sri Lankan population and to investigate whether these relationships were modified by dietary intake.
Methods
A total of 109 Sinhalese adults (61 men and 48 women aged 25–50 years) from Colombo City underwent anthropometric and biochemical measurements, dietary intake analysis, and genetic tests. Genetic risk scores (GRS) based on 10 metabolic single nucleotide polymorphisms (SNPs) (metabolic-GRS) and 10 vitamin B12 SNPs (B12-GRS) were constructed.
Results
The B12-GRS was significantly associated with serum vitamin B12 (p = 0.008) but not with metabolic traits (p > 0.05), whereas the metabolic-GRS had no effect on metabolic traits (p > 0.05) and vitamin B12 concentrations (p > 0.05). An interaction was observed between B12-GRS and protein energy intake (%) on waist circumference (p = 0.002). Interactions were also seen between the metabolic-GRS and carbohydrate energy intake (%) on waist-to-hip ratio (p = 0.015).
Conclusion
Our findings suggest that a genetically lowered vitamin B12 concentration may have an impact on central obesity in the presence of a dietary influence; however, our study failed to provide evidence for an impact of metabolic-GRS on lowering B12 concentrations. Given that our study has a small sample size, further large studies are required to confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the incidence of obesity in Sri Lanka has increased markedly [1]. The prevalence of being overweight or obese in Sri Lankan adults is 34.4% (25.2% and 9.2% in 2005 and 2006, respectively), with an upward trend being observed [1, 2]. Obesity increases the risk for certain health conditions, such as insulin resistance, diabetes mellitus, and hypertension [3]. South Asians have been observed to exhibit increased visceral fat and waist circumference (WC), hyperinsulinemia, and insulin resistance; this has been termed the “South Asian phenotype” [4]. Despite a known genetic contribution, the increase in obesity has been largely associated with changes in lifestyle habits [5, 6]. It is imperative that modifiable risk factors for obesity and associated metabolic problems are identified, especially if they can be easily addressed.
Vitamin B12 is a micronutrient that has been identified as a modifiable risk factor associated with the progression of metabolic disorders. In humans, vitamin B12 acts as an essential coenzyme involved in DNA synthesis and cellular energy production [7]. Subclinical deficiency of vitamin B12 has been linked to higher levels of homocysteine; this may have important consequences in the progression of chronic diseases, by inducing oxidative stress and inflammation [8]. Vitamin B12 deficiency has also been linked to many other complications including an increased risk of obesity [9,10,11], diabetes [12,13,14], and cardiovascular disease [15]. Currently, one study has investigated the effect of genetically instrumented vitamin B12 concentrations on body mass index (BMI) in individuals with European ancestry; however, there were no associations between the vitamin B12 genetic risk score (GRS) and BMI [16].
Genetic studies have implicated several gene loci in the predisposition to vitamin B12 deficiency, but no study has yet been carried out in the Sri Lankan population [17]. The mechanisms by which obesity and its comorbidities are related to vitamin B12 deficiency are poorly understood. Hence, we conducted a gene-based approach to explore the relationship between metabolic traits and vitamin B12 status in a Sinhalese cohort and investigated whether these relationships were modified by dietary intake in the Genetics Of Obesity and Diabetes (GOOD) study.
Study participants
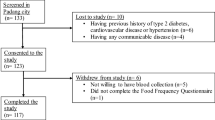
The GOOD study is a cross-sectional study that was conducted in the city of Colombo, Sri Lanka, between April and August 2017. Healthy adults between the ages of 25 and 50 years were enrolled into the study. Exclusion criteria were having a previous history of type 2 diabetes, cardiovascular disease, or hypertension, having a BMI of more than 40 kg/m2 or being classed morbidly obese by a physician, being blood related to other participants in the study, having any communicable disease, being pregnant or lactating, taking dietary or vitamin supplements, and taking medications that affect lipid metabolism or hypertension (Fig. 1).
Anthropometric measures
Body weight was measured to the nearest 100 g using an electronic scale (Seca 815, Seca GmbH. Co. kg, Germany) and height was measured to the nearest millimeter using a stadiometer (Seca 217, Seca GmbH. Co. kg, Germany). The BMI calculation was based on the body weight (kg) divided by the square of body height (m). Waist circumference and hip circumference were measured using a metal tape (Lufkin W606PM®, Parsippany, NJ, USA). Body fat percentage was estimated using a handheld bioelectrical impedance analysis technique (Omron Body Fat Monitor BF306, Omron, Milton Keynes, UK).
Biochemical analysis
Blood samples (10 ml) were collected by a trained phlebotomist in the morning, after a 12-h overnight fast. Fasting serum insulin and vitamin B12 levels were determined using the chemiluminescent microparticle immunoassay method on an Architect i1000 analyzer (Abbott Laboratories, IL, USA). Fasting plasma glucose concentrations were measured using the glucose hexokinase method using the Beckman Coulter AU5800 analyzer (Beckman Coulter®, California, USA). Glycated hemoglobin (HbA1c) was estimated by high-performance liquid chromatography using the BioRad D10 HPLC analyzer (BioRad, Hercules, CA, USA).
Dietary intake analysis
Dietary intakes were assessed using a previously validated and published [18] interviewer-administered food frequency questionnaire (FFQ) containing 85 food items. In brief, participants were asked to estimate the usual frequency (number of times per day, week, or month/never) and the portion sizes of various food items. The recorded data was analyzed with the NutriSurvey 2007 database (EBISpro, Germany) to estimate energy as well as macro- and micronutrient consumption [19].
“The Global Physical Activity Questionnaire” (GPAQ), developed by the World Health Organization (WHO), was used to measure physical activity [20]. Individuals were classified as vigorously active, when they both exercised and engaged in demanding work activities, and moderately active, when the participants either exercised or carried out heavy physical work. The remaining study participants were classified into the sedentary group.
SNP selection and genotyping
We selected 10 metabolic disease-related single nucleotide polymorphisms (SNPs) (associated with obesity and diabetes) (fat mass and obesity-associated [FTO], rs9939609 and rs8050136; melanocortin 4 receptor [MC4R], rs17782313 and rs2229616; transcription factor 7-like 2 [TCF7L2], rs12255372 and rs7903146; potassium voltage-gated channel subfamily J member 11 [KCNJ11], rs5219; calpain 10 [CAPN10], rs3792267, rs2975760, and rs5030952) for our analysis based on previously published candidate gene association and genome-wide association (GWA) studies for metabolic disease-related traits [21,22,23,24,25,26,27,28,29].
The 10 vitamin B12-related SNPs (methylenetetrahydrofolate reductase [MTHFR], rs1801133; carbamoyl-phosphate synthase 1 [CPS1], rs1047891; cubulin [CUBN], rs1801222; CD320 molecule [CD320], rs2336573; transcobalamin 2 [TCN2], rs1131603; citrate lyase beta like [CLYBL], rs41281112; fucosyltransferase 2 [FUT2], rs602662; transcobalamin 1 [TCN1], rs34324219; fucosyltransferase 6 [FUT6], rs778805 and methylmalonyl-CoA mutase [MUT], rs1141321) were chosen on the basis of the recent review article by Surendran et al. [17].
Blood samples for the measurement of DNA were transported in dry ice to the UK. Genomic DNA was extracted from a 5-ml whole blood sample from each participant and genotyping was performed at LGC Genomics (http://www.lgcgroup.com/services/genotyping), which employs the competitive allele-specific PCR-KASP® assay.
The Hardy-Weinberg equilibrium (HWE) p values were computed for the following 20 SNPs. The SNP FUT2 rs602662 and calpain 10 (CAP10) rs3792267 deviated from the HWE; however, these SNPs were not excluded from analysis. The FUT2 SNP rs602662 previously departed from HWE in a GWA study conducted in India; the authors ruled out that the deviation was not due to a genotyping error and still used this SNP for analysis in their study [30]. In addition, the KASP™ genotyping technology used in our study has been independently assessed to be over 99.8% accurate. Validation of the KASP™ genotyping was conducted at LGC genomics, where the genotyping results were assessed by two project managers separately to confirm that the data was accurate, and this ruled out genotyping artifacts as possible reasons for deviation from HWE. The reasons for deviation from HWE could be due to population or racial grouping substructure (subgrouping), non-random mating, linkage disequilibrium (incomplete mixing of different ancestral population), or chance findings [31].
Statistical analysis
The SPSS statistical package (version 22; SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Allele frequencies were estimated by gene counting (Table 1). The normality of variable distribution was verified by the Shapiro-Wilk test, and data not normally distributed were log transformed prior to analysis. We performed an independent t test to compare the means of the quantitative variables between men and women. Comparison of the means between the two groups was analyzed by the chi-square test for categorical outcomes.
A schematic representation of the study design is presented in Fig. 2. The unweighted, risk allele GRS method was calculated for each participant as the sum of risk allele counts across each SNP which predicted vitamin B12 status or metabolic disease risk. The B12-GRS was generated from the SNPs in the genes MTHFR, CPS1, CUBN, CD320, TCN2, CLYBL, FUT2, TCN1, FUT6, and MUT, which have been shown to be associated with vitamin B12 concentrations. Furthermore, another unweighted GRS was created using allele markers previously reported to be associated with metabolic disease traits. The metabolic-GRS was generated from the SNPs in the genes CAP10, KCNJ11, TCF7L2, FTO, and MC4R. A value of 0.1 or 2 was assigned to each SNP, which denotes the number of risk alleles on that SNP. These values were then calculated by adding the number of risk alleles across each SNP. The average number of risk alleles per person for the B12-GRS was 8.69 (SD = 1.70), which ranged from 5 to 15. The sample was stratified, by the median, into a “low genetic risk group,” for those with a GRS ≤ 9 risk alleles (n = 79), and into a “high genetic risk group,” for those with a GRS ≥ 10 risk alleles (n = 30). For the metabolic-GRS, the average number of risk alleles per person was 7.00 (SD = 2.28), which ranged from 1 to 13. The sample was stratified, into a “low genetic risk group,” for those with a GRS ≤ 8 risk alleles (n = 88), and into a “high genetic risk group,” for those with a GRS ≥ 9 risk alleles (n = 21). Linear regression was used to examine the association of the two GRS scores with the biochemical and anthropometric outcomes (glucose, insulin, HbAC1, vitamin B12, body fat %, BMI, WC, and waist-to-hip ratio (WHR)). The interaction between the two GRS scores and dietary factors on biochemical and anthropometric outcomes was determined by including interaction terms (GRS × diet) in the regression model. Models were adjusted for age, sex, BMI, and total energy intake, wherever appropriate.
Diagram representing the study design. The diagram shows four possible associations and four possible interactions. One-sided arrows with unbroken lines represent genetic associations and one-sided arrows with broken lines represent interactions between a lifestyle factor and GRS on serum vitamin B12/metabolic traits. We tested the association between the metabolic-GRS and vitamin B12 concentrations and metabolic disease-related traits. We then tested the associations between the B12-GRS and vitamin B12 status and metabolic disease-related traits. Lastly, we tested whether these genetic associations were modified by lifestyle factors (macronutrient intake and physical activity levels)
Correction for multiple testing was applied using Bonferroni correction [adjustment p value for association analysis was < 0.00313 [2 GRS × 8 biochemical and anthropometric outcomes (Fasting blood glucose, fasting insulin, glycated hemoglobin, vitamin B12, fat %, BMI, WC, and WHR) = 16 test)] and for interaction < 0.00078 [2 GRS × 8 biochemical and anthropometric × 4 lifestyle factors (dietary carbohydrate energy %, dietary protein energy %, dietary fat energy %, and physical activity levels)) = 64]. Given that there are no studies on GRS and no previously reported effect sizes for the South Asians, we were unable to perform a power calculation.
Results
Characteristics of the participants
In this study, 109 participants (mean age, 38.34 ± 6.92 years; BMI, 24.58 ± 4.12 kg/m2) were included. Table 2 illustrates the main characteristics of the study participants stratified according to sex. No significant difference between men and women was observed in the levels of fasting glucose, insulin, HbAC1, and plasma vitamin B12 (p > 0.05).
Association between B12-GRS and obesity GRS with biochemical and anthropometric measurements
A significant association between B12-GRS and serum vitamin B12 was observed (p = 0.008) (Supplementary Table 1 and Fig. 3). However, this finding was not significant after correction for multiple testing. No associations between the B12-GRS and metabolic traits (p > 0.05) were observed (Supplementary Table 1). Furthermore, no associations between the metabolic-GRS and vitamin B12 or metabolic traits (p > 0.05) were observed (Supplementary Table 2).
Interaction between the B12-GRS and dietary factors on biochemical and anthropometric measurements
An interaction was found between the B12-GRS and protein energy (%) on log transformed WC (p = 0.002). However, further stratification of participants based on their consumption of low, medium, and high dietary protein (energy %) did not show statistically significant associations between the GRS and the outcome in any of the tertiles, which could account for the small sample size (Supplementary Table 3).
Interaction between the metabolic-GRS and dietary factors on biochemical and anthropometric measurements
We observed a significant interaction between the metabolic-GRS and carbohydrate energy intake (%) on waist-to-hip ratio (Pinteraction = 0.015) (Fig. 4 and Table 3). Individuals who carried eight or less risk alleles for metabolic disease had 7.47% lower WHR measurements (cm) in the highest tertile of carbohydrate energy intake (%) (mean ± SD = 78.00 ± 7.90%) compared to those with nine or more risk alleles (p = 0.035) (Table 3).
Interaction between the metabolic-GRS and carbohydrate energy intake (%) on waist-to-hip ratio (cm) (Pinteraction = 0.015). Among those who consumed a high carbohydrate diet, individuals who carried nine or more risk alleles had significantly higher levels of waist-to-hip ratios compared to individuals carrying eight or less risk alleles (p = 0.035)
Interactions were also seen between the metabolic-GRS and carbohydrate energy (%) on log fasting insulin concentrations (p = 0.011) and log WC (p = 0.031) and the metabolic-GRS and protein energy (%) on log fasting insulin levels (p = 0.032) and log WC (p = 0.011) (Table 3 and Supplementary Table 3).
Interaction between the B12-GRS and physical activity on biochemical and anthropometric measurements
No statistically significant interactions were observed between the two GRSs (vitamin B12 and metabolic) and physical activity on biochemical and anthropometric measurements (Table 3 and Supplementary Table 3). After correction for multiple testing, none of these gene-diet and gene-physical activity interactions remained statistically significant.
Discussion
To our knowledge, this is the first study to use a genetic approach to explore the relationship between metabolic traits and vitamin B12 status in a South Asian population. Our study confirmed the strength of the association between B12-GRS and B12 concentrations and demonstrated the impact of genetically instrumented B12 concentrations on waist circumference, an indicator of central obesity, through the influence of dietary protein intake. Furthermore, our study has also showed a significant effect of metabolic-GRS on waist-to-hip ratio through the influence of high carbohydrate intake. Given that the total daily intake of protein is low and carbohydrate is high in Sri Lankan adults [32], our findings, if replicated in future studies, might carry significant public health implications in terms of revising the food-based dietary guidelines which could prevent central obesity and the associated CVD-related outcomes.
In this study, we constructed a GRS consisting of ten vitamin B12 decreasing SNPs in genes involved in vitamin B12 metabolism [17]. The B12-GRS was associated with vitamin B12 levels, suggesting that it would be an ideal instrument for vitamin B12 status. Given the lack of association between the B12-GRS and metabolic disease traits in our study, we were unable to provide evidence for linear decreases in vitamin B12 concentrations having substantive effects on metabolic disease traits. However, we found a significant interaction between the B12-GRS and protein energy (%) on log WC. Interestingly, individuals who carried nine or less alleles had lower WC when consuming a high protein diet compared to those consuming a low protein diet. Although no statistically significant differences in WC were observed between the alleles of the B12-GRS, the impact of the B12-GRS on WC was observed only under the influence of a high protein diet. Further investigations are required to confirm this finding to determine the clinical significance and potential applications as part of weight management interventions.
At present, carbohydrates constitute the majority of the energy intake among South Asian countries such as Sri Lanka (~ 71.2%) [32]; in contrast, the consumption of carbohydrates is lower in Western countries (~ 45%) [33]. Furthermore, high carbohydrate intake has been associated with an increased risk of diabetes in a South Indian population [34] and an increase in WC among premenopausal (20–45 years) Sri Lankan women [35]. In the present study, we found a significant interaction between the metabolic-GRS and carbohydrate energy percentage on waist-to-hip ratio, where the individuals carrying more than nine risk alleles had a higher waist-to-hip ratio among those in the highest tertile of carbohydrate energy percentage. There are no previous reports of the risk variants used in our GRS, but Goni et al. [36] found that carbohydrates (total and complex) interacted with a GRS of 16 obesity/lipid metabolism polymorphisms to modify the effect on body fat mass in 711 individuals of Caucasian ancestry. In our study, we only observed interactions of the metabolic-GRS on WC and waist-to-hip ratio, which suggests that effects are likely to be on central obesity as opposed to common obesity.
South Asians have a higher risk of developing obesity-related non-communicable diseases relative to white Caucasians despite lower BMI levels; this has been termed the “South Asian phenotype.” The distinctive features of this phenotype include a higher WC, abdominal adiposity combined with insulin resistance, and a greater predisposition to diabetes [4]. The role of vitamin B12 in promoting this adverse phenotype has been suggested by Yajnik et al., who demonstrated that offspring born to mothers with a low vitamin B12 and high folate status had a greater risk of developing insulin resistance during childhood [12]. According to Yajnik et al., vitamin B12 deficiency prevents the generation of tetrahydrofolate from 5-methyltetrahydrofolate in the one-carbon metabolism cycle; as a result, homocysteine levels accumulate leading to altered lean tissue deposition and reduced protein synthesis [12]. Furthermore, vitamin B12 is involved in the conversion of methylmalonyl-CoA to succinyl-CoA by the enzyme methylmalonyl-CoA mutase (adenosyl-B12 as a cofactor). Subsequently, vitamin B12 deficiency results in elevated methylmalonyl-CoA, inhibiting the mitochondrial enzyme carnitine palmitoyltransferase, which may promote lipogenesis and insulin resistance [12, 37].
No studies to date have investigated interactions between the two GRSs and physical activity on metabolic traits and B12 concentrations in Asian Sri Lankans. Although 60% of Sri Lankan adults are reported to be highly physically active [38], no significant interactions were found between the two GRSs and physical activity on metabolic traits, which could be due to a small sample size and measurement bias associated with self-reported physical activity questionnaire. The strengths of our study include the use of a validated food frequency questionnaire [18] to measure macronutrient intake, the comprehensive measurements of lifestyle factors, and the use of GRSs which increased the statistical power of our study [39]. Nevertheless, some limitations need to be acknowledged. The first limitation concerns the relatively small sample size of the study; however, we were still able to identify significant gene-diet interactions. Furthermore, we used Bonferroni correction to correct for multiple testing and this can often lead to larger power, specifically where studies have a small sample size and a small number of disease-associated markers. This is also true for when studies have a large allele frequency difference due to a small sample size [40]. Secondly, information about the type of oil used for frying, the estimation of different dietary fat components (monounsaturated or saturated fatty acids), and vitamin B12 intake was not collected. This could have limited our in-depth analysis of interactions of specific macronutrients and vitamins with the two GRSs. Furthermore, the study was limited to Sinhalese adults in Colombo, and the conclusions may not be applicable to other ethnic groups in Sri Lanka. Finally, none of the genetic associations or gene-lifestyle interactions were statistically significant after correction for multiple testing; however, given that this is the first study using a genetic approach to establish a relationship between vitamin B12 status and metabolic disease outcomes in South Asians, we have taken into consideration the significant findings; hence, further large studies are required to replicate our findings.
In summary, our study suggests that a genetically lowered vitamin B12 concentration may have an impact on central obesity in the presence of a dietary influence; however, our study failed to show an impact of the metabolic-GRS on lowering B12 concentrations through a dietary influence. Our study also showed a significant effect of the metabolic-GRS on waist-to-hip ratio, another indicator of central obesity, through the influence of a high carbohydrate intake. However, after correction for multiple testing, none of these findings were statistically significant. Hence, further replication studies are highly warranted on large samples to confirm or refute our findings.
Data availability
Data from this project will not be shared because additional results from the study are yet to be published.
Abbreviations
- SNPs:
-
Single nucleotide polymorphisms
- MTHFR :
-
Methylenetetrahydrofolate reductase
- CPS1 :
-
Carbamoyl-phosphate synthase 1
- CUBN :
-
Cubulin
- CD320 :
-
CD320 molecule
- TCN2 :
-
Transcobalamin 2
- CLYBL :
-
Citrate lyase beta like
- FUT2 :
-
Fucosyltransferase 2
- TCN1 :
-
Transcobalamin 1
- FUT6 :
-
Fucosyltransferase 6
- MUT :
-
Methylmalonyl-CoA mutase
- CAP10 :
-
Calpain 10
- KCNJ11 :
-
Potassium voltage-gated channel subfamily J member 11
- TCF7L2 :
-
Transcription factor 7-like 2
- FTO :
-
Fat mass and obesity-associated
- MC4R :
-
Melanocortin 4 receptor
- BMI:
-
Body mass index
- SD:
-
Standard deviations
- WC:
-
Waist circumference
- WHR:
-
Waist-to-hip ratio
References
Jayawardena R, Byrne NM, Soares MJ, Katulanda P, Hills AP. The obesity epidemic in Sri Lanka revisited. Asia Pacific Journal of Public Health. 2015;27(2):NP1298–9.
Katulanda P, Jayawardena MAR, Sheriff MHR, Constantine GR, Matthews DR. Prevalence of overweight and obesity in Sri Lankan adults. Obes Rev. 2010;11(11):751–6.
Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. Jama. 1999;282(16):1523–9.
Mohan V, Deepa R. Adipocytokines and the expanding 'Asian Indian phenotype. J Assoc Physicians India. 2006;54:685–6.
Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15(1):131.
Hruby A, Hu FB. The epidemiology of obesity: a big picture. PharmacoEconomics. 2015;33(7):673–89.
O’Leary F, Samman S. Vitamin B(12) in health and disease. Nutrients. 2010;2(3):299–316.
Qureshi GA, et al. Is the deficiency of vitamin B12 related to oxidative stress and neurotoxicity in Parkinson's patients? CNS Neurol Disord Drug Targets. 2008;7(1):20–7.
Pinhas-Hamiel O, Doron-Panush N, Reichman B, Nitzan-Kaluski D, Shalitin S, Geva-Lerner L. Obese children and adolescents: a risk group for low vitamin B12 concentration. Arch Pediatr Adolesc Med. 2006;160(9):933–6.
MacFarlane AJ, Greene-Finestone LS, Shi Y. Vitamin B-12 and homocysteine status in a folate-replete population: results from the Canadian Health Measures Survey. Am J Clin Nutr. 2011;94(4):1079–87.
Baltaci D, et al. Association of vitamin B12 with obesity, overweight, insulin resistance and metabolic syndrome, and body fat composition; primary care-based study. Med Glas (Zenica). 2013;10(2):203–10.
Yajnik CS, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51(1):29–38.
Krishnaveni GV, Hill JC, Veena SR, Bhat DS, Wills AK, Karat CLS, et al. Low plasma vitamin B12 in pregnancy is associated with gestational 'diabesity' and later diabetes. Diabetologia. 2009;52(11):2350–8.
Knight BA, Shields BM, Brook A, Hill A, Bhat DS, Hattersley AT, et al. Lower circulating B12 is associated with higher obesity and insulin resistance during pregnancy in a non-diabetic White British population. PLoS One. 2015;10(8):e0135268.
Weikert C, Dierkes J, Hoffmann K, Berger K, Drogan D, Klipstein-Grobusch K, et al. B vitamin plasma levels and the risk of ischemic stroke and transient ischemic attack in a German cohort. Stroke. 2007;38(11):2912–8.
Allin KH et al. Genetic determinants of serum vitamin B12 and their relation to body mass index. Eur J Epidemiol. 2016;1–10.
Surendran S, et al. An update on vitamin B12-related gene polymorphisms and B12 status. Genes Nutr. 2018;13:2.
Jayawardena R, et al. Validity of a food frequency questionnaire to assess nutritional intake among Sri Lankan adults. SpringerPlus. 2016;5:162.
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S discussion 1229S-1231S.
Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health. 2006;14(2):66–70.
Illangasekera YA, et al. Association of FTO and near MC4R variants with obesity measures in urban and rural dwelling Sri Lankans. Obes Res Clin Pract. 2016;10(Suppl 1):S117–s124.
Ramya K, Radha V, Ghosh S, Majumder PP, Mohan V. Genetic variations in the FTO gene are associated with type 2 diabetes and obesity in south Indians (CURES-79). Diabetes Technol Ther. 2011;13(1):33–42.
Uma Jyothi K, Jayaraj M, Subburaj KS, Prasad KJ, Kumuda I, Lakshmi V, et al. Association of TCF7L2 gene polymorphisms with T2DM in the population of Hyderabad, India. PLoS One. 2013;8(4):e60212.
Bodhini D, Gaal S, Shatwan I, Ramya K, Ellahi B, Surendran S, et al. Interaction between TCF7L2 polymorphism and dietary fat intake on high density lipoprotein cholesterol. PLoS One. 2017;12(11):e0188382.
Kommoju UJ, Maruda J, Kadarkarai Samy S, Irgam K, Kotla JP, Reddy BM. Association of IRS1, CAPN10, and PPARG gene polymorphisms with type 2 diabetes mellitus in the high-risk population of Hyderabad, India. J Diabetes. 2014;6(6):564–73.
Adak S, Sengupta S, Chowdhury S, Bhattacharyya M. Co-existence of risk and protective haplotypes of Calpain 10 gene to type 2 diabetes in the eastern Indian population. Diab Vasc Dis Res. 2010;7(1):63–8.
Loos RJF. The genetic epidemiology of melanocortin 4 receptor variants. Eur J Pharmacol. 2011;660(1):156–64.
Weedon MN, Schwarz PEH, Horikawa Y, Iwasaki N, Illig T, Holle R, et al. Meta-analysis and a large association study confirm a role for calpain-10 variation in type 2 diabetes susceptibility. Am J Hum Genet. 2003;73(5):1208–12.
Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52(2):568–72.
Nongmaithem SS, Joglekar CV, Krishnaveni GV, Sahariah SA, Ahmad M, Ramachandran S, et al. GWAS identifies population specific new regulatory variants in FUT6 associated with plasma B12 concentrations in Indians. Hum Mol Genet. 2017;26:2551–64.
Leonard DGB. Molecular pathology in clinical practice. Springer International Publishing; 2016.
Jayawardena R, Thennakoon S, Byrne N, Soares M, Katulanda P, Hills A. Energy and nutrient intakes among Sri Lankan adults. Int Arch Med. 2014;7:34.
Stevens J, Ahn K, Juhaeri, Houston D, Steffan L, Couper D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: the ARIC study. Diabetes Care. 2002;25(10):1715–21.
Mohan V, Radhika G, Sathya RM, Tamil SR, Ganesan A, Sudha V. Dietary carbohydrates, glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59). Br J Nutr. 2009;102(10):1498–506.
Rathnayake KM, Roopasingam T, Dibley MJ. High carbohydrate diet and physical inactivity associated with central obesity among premenopausal housewives in Sri Lanka. BMC Res Notes. 2014;7:564.
Goni L, Cuervo M, Milagro FI, Martínez JA. A genetic risk tool for obesity predisposition assessment and personalized nutrition implementation based on macronutrient intake. Genes Nutr. 2015;10(1):445.
Ruderman NB, Saha AK, Kraegen EW. Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology. 2003;144(12):5166–71.
Katulanda P, Jayawardana R, Ranasinghe P, Rezvi Sheriff MH, Matthews DR. Physical activity patterns and correlates among adults from a developing country: the Sri Lanka Diabetes and Cardiovascular Study. Public Health Nutr. 2013;16(9):1684–92.
Hüls A, et al. Comparison of weighting approaches for genetic risk scores in gene-environment interaction studies. BMC Genet. 2017;18:115.
Zou G, Zuo Y. On the sample size requirement in genetic association tests when the proportion of false positives is controlled. Genetics. 2006;172(1):687–91.
Acknowledgments
We are grateful to the study participants for their cooperation and participation. We thank Modera Police Station and Rohan Pelpola for their contributions to the recruitment of participants. We also thank Suranga Singhapura, Padmini Dassanayake, Sunethra Wickramarathne, Ransi Perera, Sumathe Thanabalasingam, Umesh Thanabalasingam, Osanda Pelpola, Krishanthi Pelpola, Divyanga Molagoda, Gumesha Thilakarathne, Shinoli Wickramaratne, Nuwansamitha Fernando, Lakmali Fernando, Samitha Hettiarachchi, Bhanuka Pathberiya, Thejana Pathberiya, Kasun Perera, Vinul Perera, Kalum Jayathilake, Yamuna Jayathilaka, Wasantha Kahapalaarachichi, Ashintha Perera, and Neeliya De Saram for their help in data collection. We also thank Dr. Suresh Surendran for the contributions for the preparation of the study. We would like to thank Dr. Seevali Surendran for the Sinhalese language editing.
Funding
We would like to thank the Farnborough College of Technology for contributing travel expenses towards the project. We also acknowledge the support for the GeNuIne Collaboration from the British Nutrition Foundation.
Author information
Authors and Affiliations
Contributions
SS and KSV drafted the manuscript; SS performed the statistical analysis; and SS, VKS, DJA, and RL were responsible for the study conception. KW provided guidance to the research; SS and RL conducted data and sample collection; SS, RJ, and SA were involved in the dietary data analysis; SS and SaS were involved in the physical activity data analysis; KSV designed the gene-diet interaction study; and SS, VKS, JAL, and RJ critically reviewed the manuscript. All authors contributed to and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Review Committee of the University of Colombo (EC-17-107) and the University of Reading Research Ethics Committee (17/25). All participants signed informed consent prior to their participation.
Consent for publication
Not applicable.
Competing interests
All authors declare that there is no conflict of interest associated with their contribution to this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Surendran, S., Alsulami, S., Lankeshwara, R. et al. A genetic approach to examine the relationship between vitamin B12 status and metabolic traits in a South Asian population. Int J Diabetes Dev Ctries 40, 21–31 (2020). https://doi.org/10.1007/s13410-019-00749-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-019-00749-8