Abstract
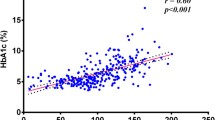
Diabetes is a metabolic disorder that is increasing in prevalence at a very fast pace in developing countries such as South Africa. There is a strong link between diabetes and cardiovascular disease with studies showing that diabetic patients are two to four times more likely to suffer from cardiovascular disease than healthy people. The dysregulation of lipid and lipoprotein metabolism and the subsequent oxidation of low-density lipoprotein (LDL) is one of the major reasons for the increased cardiovascular risk associated with diabetes. The main objective of this study was to analyze the concentrations of oxidized LDL in diabetic patients and to uncover factors associated with oxidized LDL. In total, 67 type 2 diabetic mellitus patients and 48 healthy control participants participated in the cross-sectional study. Oxidized LDL concentration in serum and the total antioxidant status were determined by ELISA, while the size of LDL and high-density lipoprotein (HDL) particles were determined by gradient gel electrophoresis. The concentration of oxidized LDL was significantly elevated in the diabetic patients. The diabetic patients also had a significantly higher percentage of small dense LDL and a higher concentration of triglycerides than the control group, while their peak LDL size and the total antioxidant capacity were significantly lower than those in the control group. The percentage of small dense LDL, triglycerides, peak LDL size, and total antioxidant capacity also varied significantly across the quartiles of oxidized LDL in the entire study population. Multiple regression analysis revealed that triglyceride and glycated hemoglobin were the most important parameters to be independently associated with oxidized LDL concentrations. The diabetic patients had high concentrations of small dense LDL and oxidized LDL. The concentrations of the highly atherogenic oxidized LDL particles were found to be independently associated with the concentrations of triglycerides and glycated hemoglobin. This indicated that proper diabetic control could reduce the risk of cardiovascular disease in these individuals.
Similar content being viewed by others
References
Shaw JE, Sicree RA, Zimmet PZ. Global estimates for the prevalence of diabetes 2010 and 2030. Diabetes Res Clin Pr. 2010;87(1):4–14.
Peer N, Steyn K, Lombard C, Vythilingum B, Levitt NS. Rising diabetes prevalence among urban-dwelling black South Africans. PLoS One. 2012;7(9):e43336.
Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms. Diabetes Care. 2010;33(2):442–9.
Nakhjavani M, Khajeali L, Esteghamati A, Morteza A, Jamali A, Dadkhahipour S. Serum oxidized-LDL is associated with diabetes duration independent of maintaining optimized levels of LDL-cholesterol. Lipids. 2010;45(4):321–7.
Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272(34):20963–6.
Rajman I, Eacho PI, Chowienczyk PJ, Ritter JM. LDL particle size: an important drug target? Br J Clin Pharmacol. 1999;48(2):125–33.
Ionica F, Popescu F, Pisoschi C, Gofita E. Clinical consequence of the physicochemical properties of LDL particles in type 2 diabetes. Curr Health Sci J. 2009;35(1):73–8.
Yoshida H, Kisugi R. Mechanism of LDL oxidation. Clinica Chemica Acta. 2010;411(23–24):1875–82.
Li D, Mehta JL. Oxidized LDL, a critical factor in atherogenesis. Cardiovasc Res. 2005;68(3):353–4.
Singh AT, Rainwater DL, Haffner SM, Vande Berg JL, Shelledy WR, Moore PH, Moore PH Jr, Dyer TD. Effect of diabetes on lipoprotein size. Arterioscler Thromb Vasc Biol. 1995;15(11):1805–11.
Vasquez G, Duva S, Jacobs RD Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–28.
Cassano PA, Rosner B, Vokonas PS, Weiss ST. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus: a prospective cohort study of men in the normative aging study. Am J Epidemiol. 1992;136(12):1474–86.
Welborn TA, Dhaliwal SS. Preferred clinical measures of central obesity for predicting mortality. Eur J Clin Nutr. 2007;61(12):1373–9.
Srikanthan P, Seeman TE, Karlamangla AS. Waist-hip-ratio as a predictor of all-cause mortality in high-functioning older adults. Ann Epidemiol. 2009;19:724–31.
Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:150–9.
Duvillard L, Pont F, Florentin E, Galland-Jos C, Gambert P, Vergès B. Metabolic abnormalities of apolipoprotein B-containing lipoproteins in non-insulin-dependent diabetes: a stable isotope kinetic study. Eur J Clin Investig. 2000;30:685–94.
Karimian NP, Adeli K. Insulin silences apolipoprotein B mRNA translation by inducing intracellular traffic into cytoplasmic RNA granules. Biochemistry. 2011;50(32):6942–50.
Yanai H, Hirowatari Y, Ito K, Kurosawa H, Tada N, Yoshida H. Understanding of diabetic dyslipidemia by using tJ Clin Med Reshe anion-exchange high performance liquid chromatography data. 2016;8(5):424–426.
Au WS, Kung HF, Lin MC. Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells. Diabetes. 2003;52(5):1073–80.
Hayek T, Azrolan N, Verdery RB, Walsh A, Chajek-Shaul T, Agellon LB, Breslow JL. Hypertriglyceridemia and cholesteryl ester transfer protein interact to dramatically alter high density lipoprotein levels, particle sizes, and metabolism. Studies in transgenic mice. J Clin Invest. 1993;92(3):1143–52.
Rammadan SS, Cevik C, Quobaili FA. Impact of type 2 diabetes on serum CETP levels. IJPSRR. 2014;26, 1,15:98–100.
Kiranmayi PV, Vivekanand N. Pandit Vl. The study of lipid profile and oxidised-LDL in type 2 diabetes mellitus. SJAMS. 2014;2(3D):1119–22.
Superko RH. Advanced lipoprotein testing and subfraction are clinically useful. Circulation. 2009;119:2383–95.
Adiels M, Olofson SO, Taskinen MR, Boren J. Overproduction of very low-density ipoproteins is the hallmark of the dyslipidemia in metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:629–36.
EEMN E, Almarzouki A, Assiri AM, Omebea M, Babakr AT. Oxidized low density lipoprotein and total antioxidant capacity in type-2 diabetic and impaired glucose tolerance in Saudi men. Diabetol Metab Syndr. 2014;6:94.
Wang J, Gu Q, Li K, Zhang C. CETP and oxidized LDL levels increase in dyslipidemic subjects. Clin Biochem. 2007;40:995–9.
Sobal G, Menzel J, Sinzinger H. Why is glycated LDL more susceptible to oxidation than native LDL? A comparative study. Prostaglandins Leukot Essent Fatty Acids. 2000;63(4):177–86.
Opara EC, Rahman EA, Soliman S, Kamel WA, Souka S, Lowe JE, Aleem SA. Depletion of total antioxidants in type 2 diabetes. Metabolism. 1999;48(11):1414–7.
Ceriello A, Bortolotti N, Pirisi M, Crescentitni A, Tonutti L, Motz E, Russo A, Glaomello R, Stel G, Taboga C. Total antioxidant capacity predicts thrombosis-prone status in NIDDM patients. Diabetes Care. 1997;20(10):1589–93.
Mertens A, Holvoet P. Oxidized LDL and HDL: antagonists in atherothromobosis. FASEB J. 2001;15(12):2073–84.
Farbstein D, Levy AP. HDL dysfunction in diabetes: causes and possible treatments. Expert Rev Cardiovasc Ther. 2012;10(3):353–61.
Dhanunjaya Y, Vijaya D, Dolia PB. Decreased basal activity of HDL associated enzyme: paraoxonase (PON) during uncompensated oxidative stress among type 2 diabetes mellitus patients. Int J Diabetes Dev Ctries. 2015;35(3):483–290.
Acknowledgements
The authors would like to thank all the research participants who took part in this study, and Walter Sisulu University, South Africa, for funding this study.
Author information
Authors and Affiliations
Contributions
Jim Joseph: Study design; data acquisition; data analysis; statistical analysis; manuscript preparation
Farzana Ganjifrockwala: Data acquisition; manuscript editing
Grace George: Study design; statistical analysis; manuscript editing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study conformed to the 1964 Helsinki declaration and its later amendments. The study was approved by the Health Research Ethics and Bio-Safety Committee of Walter Sisulu University (Protocol No: 013/012).
Statement of informed consent
Written informed consent was obtained from all the participants included in the study.
Funding
This study was funded by the research department of Walter Sisulu University, South Africa.
Rights and permissions
About this article
Cite this article
Joseph, J.T., Ganjifrockwala, F. & George, G. Serum oxidized LDL and the factors associated with LDL oxidation in black South African type 2 diabetes mellitus patients. Int J Diabetes Dev Ctries 38, 75–79 (2018). https://doi.org/10.1007/s13410-017-0559-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-017-0559-0