Abstract
Background
Malignant peripheral nerve sheath tumors (MPNSTs) are aggressive sarcomas that typically develop in the setting of neurofibromatosis type 1 (NF1) and cause significant morbidity. Conventional therapies are often ineffective for MPNSTs. Ribonucleotide reductase subunit M2 (RRM2) is involved in DNA synthesis and repair, and is overexpressed in multiple cancers. However, its role in NF1-associated MPNSTs remains unknown. Our objective was to determine the therapeutic and prognostic potential of RRM2 in NF1-associated MPNSTs.
Methods
Identification of hub genes was performed by using NF1-associated MPNST microarray datasets. We detected RRM2 expression by immunochemical staining in an MPNST tissue microarray, and assessed the clinical and prognostic significance of RRM2 in an MPNST cohort. RRM2 knockdown and the RRM2 inhibitor Triapine were used to assess cell proliferation and apoptosis in NF1-associated MPNST cells in vitro and in vivo. The underlying mechanism of RRM2 in NF1-associated MPNST was revealed by transcriptome analysis.
Results
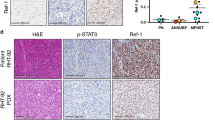
RRM2 is a key hub gene and its expression is significantly elevated in NF1-associated MPNST. We revealed that high RRM2 expression accounted for a larger proportion of NF1-associated MPNSTs and confirmed the correlation of high RRM2 expression with poor overall survival. Knockdown of RRM2 inhibited NF1-associated MPNST cell proliferation and promoted apoptosis and S-phase arrest. The RRM2 inhibitor Triapine displayed dose-dependent inhibitory effects in vitro and induced significant tumor growth reduction in vivo in NF1-associated MPNST. Analysis of transcriptomic changes induced by RRM2 knockdown revealed suppression of the AKT-mTOR signaling pathway. Overexpression of RRM2 activates the AKT pathway to promote NF1-associated MPNST cell proliferation.
Conclusions
RRM2 expression is significantly elevated in NF1-associated MPNST and that high RRM2 expression correlates with poorer outcomes. RRM2 acts as an integral part in the promotion of NF1-associated MPNST cell proliferation via the AKT-mTOR signaling pathway. Inhibition of RRM2 may be a promising therapeutic strategy for NF1-associated MPNST.







Similar content being viewed by others
Data availability
The raw sequencing data generated in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/geo/GSE213988.
Abbreviations
- NF1:
-
Neurofibromatosis Type 1
- MPNST:
-
Malignant Peripheral Nerve Sheath Tumor
- RRM2:
-
Ribonucleotide reductase subunit M2
- STS:
-
Soft-tissue sarcoma; plexiform neurofibromas (PNF)
- RNR:
-
Ribonucleotide reductase
- dNTP:
-
Deoxyribonucleotides
- HNSCC:
-
Head and neck squamous cell carcinoma
- DEG:
-
Differentially expressed gene
- qPCR:
-
Quantitative Polymerase Chain Reaction
- RNA-seq:
-
RNA sequencing
- SD:
-
Standard deviation
- CHK1:
-
Checkpoint Kinase 1
References
L.M.N. Wu, Y. Deng, J. Wang, C. Zhao, J. Wang, R. Rao, L. Xu, W. Zhou, K. Choi, T.A. Rizvi et al., Programming of Schwann cells by Lats1/2-TAZ/YAP signaling drives malignant peripheral nerve sheath tumorigenesis. Cancer Cell 33, (2018). https://doi.org/10.1016/j.ccell.2018.01.005
V. Kochat, A.T. Raman, S.M. Landers, M. Tang, J. Schulz, C. Terranova, J.P. Landry, A.D. Bhalla, H.C. Beird, C.-C. Wu et al., Enhancer reprogramming in PRC2-deficient malignant peripheral nerve sheath tumors induces a targetable de-differentiated state. Acta Neuropathol. 142, 565–590 (2021). https://doi.org/10.1007/s00401-021-02341-z
K.L. Watson, G.A. Al Sannaa, C.M. Kivlin, D.R. Ingram, S.M. Landers, C.L. Roland, J.N. Cormier, K.K. Hunt, B.W. Feig, B. Ashleigh Guadagnolo et al., Patterns of recurrence and survival in sporadic, neurofibromatosis Type 1-associated, and radiation-associated malignant peripheral nerve sheath tumors. J. Neurosurg. 126, 319–329 (2017). https://doi.org/10.3171/2015.12.JNS152443
D.T. Miller, D. Freedenberg, E. Schorry, N.J. Ullrich, D. Viskochil and B.R. Korf, Health supervision for children with neurofibromatosis type 1. Pediatrics 143, (2019). https://doi.org/10.1542/peds.2019-0660
D. Bradford, A. Kim, Current treatment options for malignant peripheral nerve sheath tumors. Curr. Treat. Options Oncol. 16, 328 (2015). https://doi.org/10.1007/s11864-015-0328-6
A. Hassan, R.C. Pestana, A. Parkes, Systemic options for malignant peripheral nerve sheath tumors. Curr. Treat. Options Oncol. 22, 33 (2021). https://doi.org/10.1007/s11864-021-00830-7
B.L. Greene, G. Kang, C. Cui, M. Bennati, D.G. Nocera, C.L. Drennan, J. Stubbe, Ribonucleotide reductases: structure, chemistry, and metabolism suggest new therapeutic targets. Annu. Rev. Biochem. 89, 45–75 (2020). https://doi.org/10.1146/annurev-biochem-013118-111843
M. Zhang, Y. Weng, Z. Cao, S. Guo, B. Hu, M. Lu, W. Guo, T. Yang, C. Li, X. Yang, Y. Huang, ROS-Activatable siRNA-Engineered Polyplex for NIR-Triggered synergistic cancer treatment. ACS Appl. Mater. Interfaces 12, 32289–32300 (2020). https://doi.org/10.1021/acsami.0c06614
V. D’Angiolella, V. Donato, F.M. Forrester, Y.-T. Jeong, C. Pellacani, Y. Kudo, A. Saraf, L. Florens, M.P. Washburn, M. Pagano, Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell 149, 1023–1034 (2012). https://doi.org/10.1016/j.cell.2012.03.043
C. Nunes, L. Depestel, L. Mus, K.M. Keller, L. Delhaye, A. Louwagie, M. Rishfi, A. Whale, N. Kara, S.R. Andrews et al., RRM2 enhances MYCN-driven neuroblastoma formation and acts as a synergistic target with CHK1 inhibition. Sci. Adv. 8, eabn1382 (2022). https://doi.org/10.1126/sciadv.abn1382
B. Tang, W. Xu, Y. Wang, J. Zhu, H. Wang, J. Tu, Q. Weng, C. Kong, Y. Yang, R. Qiu, Z. Zhao, M. Xu, J. Ji, Identification of critical ferroptosis regulators in lung adenocarcinoma that RRM2 facilitates tumor immune infiltration by inhibiting ferroptotic death. Clin. Immunol. 232, 108872 (2021). https://doi.org/10.1016/j.clim.2021.108872
Q. Liu, L. Guo, H. Qi, M. Lou, R. Wang, B. Hai, K. Xu, L. Zhu, Y. Ding, C. Li, L. Xie, J. Shen, X. Xiang, J. Shao, A MYBL2 complex for RRM2 transactivation and the synthetic effect of MYBL2 knockdown with WEE1 inhibition against colorectal cancer. Cell Death Dis. 12, 683 (2021). https://doi.org/10.1038/s41419-021-03969-1
C. Kretschmer, A. Sterner-Kock, F. Siedentopf, W. Schoenegg, P.M. Schlag, W. Kemmner, Identification of early molecular markers for breast cancer. Mol. Cancer 10, 15 (2011). https://doi.org/10.1186/1476-4598-10-15
S. Zheng, X. Wang, Y.-H. Weng, X. Jin, J.-L. Ji, L. Guo, B. Hu, N. Liu, Q. Cheng, J. Zhang, H. Bai, T. Yang, X.-H. Xia, H.-Y. Zhang, S. Gao, Y. Huang, siRNA knockdown of RRM2 effectively suppressed pancreatic tumor growth alone or synergistically with doxorubicin. Mol. Ther. Nucleic Acids 12, 805–816 (2018). https://doi.org/10.1016/j.omtn.2018.08.003
X. Xu, J.L. Page, J.A. Surtees, H. Liu, S. Lagedrost, Y. Lu, R. Bronson, E. Alani, A.Y. Nikitin, R.S. Weiss, Broad overexpression of ribonucleotide reductase genes in mice specifically induces lung neoplasms. Cancer Res. 68, 2652–2660 (2008). https://doi.org/10.1158/0008-5472.CAN-07-5873
C. Li, J. Zheng, S. Chen, B. Huang, G. Li, Z. Feng, J. Wang, S. Xu, RRM2 promotes the progression of human glioblastoma. J. Cell Physiol. 233, 6759–6767 (2018). https://doi.org/10.1002/jcp.26529
M.A. Rahman, A.R.M.R. Amin, D. Wang, L. Koenig, S. Nannapaneni, Z. Chen, Z. Wang, G. Sica, X. Deng, Z.G. Chen, D.M. Shin, RRM2 regulates Bcl-2 in head and neck and lung cancers: a potential target for cancer therapy. Clin. Cancer Res. 19, 3416–3428 (2013). https://doi.org/10.1158/1078-0432.CCR-13-0073
S.E. Huff, J.M. Winter, C.G. Dealwis, Inhibitors of the cancer target ribonucleotide reductase, past and present. Biomolecules 12, (2022). https://doi.org/10.3390/biom12060815
D. Szklarczyk, A.L. Gable, K.C. Nastou, D. Lyon, R. Kirsch, S. Pyysalo, N.T. Doncheva, M. Legeay, T. Fang, P. Bork, L.J. Jensen, C. von Mering, The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49, D605–D612 (2021). https://doi.org/10.1093/nar/gkaa1074
P. Shannon, A. Markiel, O. Ozier, N.S. Baliga, J.T. Wang, D. Ramage, N. Amin, B. Schwikowski, T. Ideker, Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003)
C.-H. Chin, S.-H. Chen, H.-H. Wu, C.-W. Ho, M.-T. Ko, C.-Y. Lin, cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 8 Suppl 4, S11 (2014). https://doi.org/10.1186/1752-0509-8-S4-S11
C. Guzmán, M. Bagga, A. Kaur, J. Westermarck, D. Abankwa, ColonyArea: an ImageJ plugin to automatically quantify colony formation in clonogenic assays. PLoS One 9, e92444 (2014). https://doi.org/10.1371/journal.pone.0092444
M. Martin, Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal (2011). https://doi.org/10.14806/ej.17.1.200
D. Kim, B. Langmead, S.L. Salzberg, HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015). https://doi.org/10.1038/nmeth.3317
M. Pertea, G.M. Pertea, C.M. Antonescu, T.-C. Chang, J.T. Mendell, S.L. Salzberg, StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015). https://doi.org/10.1038/nbt.3122
M.I. Love, W. Huber, S. Anders, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014)
X. Liu, H. Zhang, L. Lai, X. Wang, S. Loera, L. Xue, H. He, K. Zhang, S. Hu, Y. Huang, R.A. Nelson, B. Zhou, L. Zhou, P. Chu, S. Zhang, S. Zheng, Y. Yen, Ribonucleotide reductase small subunit M2 serves as a prognostic biomarker and predicts poor survival of colorectal cancers. Clin. Sci. (Lond) 124, 567–578 (2013). https://doi.org/10.1042/CS20120240
L.-M. Wang, F.-F. Lu, S.-Y. Zhang, R.-Y. Yao, X.-M. Xing, Z.-M. Wei, Overexpression of catalytic subunit M2 in patients with ovarian cancer. Chin. Med. J. (Engl) 125, 2151–2156 (2012)
A. Pemov, H. Li, W. Presley, M.R. Wallace, D.T. Miller, Genetics of human malignant peripheral nerve sheath tumors. Neurooncol. Adv. 2, i50–i61 (2020). https://doi.org/10.1093/noajnl/vdz049
J.F. Longo, S.M. Weber, B.P. Turner-Ivey, S.L. Carroll, Recent advances in the diagnosis and pathogenesis of neurofibromatosis Type 1 (NF1)-associated peripheral nervous system neoplasms. Adv. Anat. Pathol. 25, 353–368 (2018). https://doi.org/10.1097/PAP.0000000000000197
L.-L. Ge, M.-Y. Xing, H.-B. Zhang, Q.-F. Li, Z.-C. Wang, Role of nerves in neurofibromatosis type 1-related nervous system tumors. Cell Oncol. (Dordr) 45, 1137–1153 (2022). https://doi.org/10.1007/s13402-022-00723-3
X. Jiang, Y. Li, N. Zhang, Y. Gao, L. Han, S. Li, J. Li, X. Liu, Y. Gong, C. Xie, RRM2 silencing suppresses malignant phenotype and enhances radiosensitivity via activating cGAS/STING signaling pathway in lung adenocarcinoma. Cell Biosci. 11, 74 (2021). https://doi.org/10.1186/s13578-021-00586-5
M. Endo, H. Yamamoto, N. Setsu, K. Kohashi, Y. Takahashi, T. Ishii, K.-I. Iida, Y. Matsumoto, M. Hakozaki et al., Prognostic significance of AKT/mTOR and MAPK pathways and antitumor effect of mTOR inhibitor in NF1-related and sporadic malignant peripheral nerve sheath tumors. Clin. Cancer Res. 19, 450–461 (2013). https://doi.org/10.1158/1078-0432.CCR-12-1067
S. Nagabushan, L.M.S. Lau, P. Barahona, M. Wong, A. Sherstyuk, G.M. Marshall, V. Tyrrell, E.A. Wegner, P.G. Ekert, M.J. Cowley, C. Mayoh, T.N. Trahair, P. Crowe, A. Anazodo, D.S. Ziegler, Efficacy of MEK inhibition in a recurrent malignant peripheral nerve sheath tumor. NPJ Precis. Oncol. 5, 9 (2021). https://doi.org/10.1038/s41698-021-00145-8
C. Gregorian, J. Nakashima, S.M. Dry, P.L. Nghiemphu, K.B. Smith, Y. Ao, J. Dang, G. Lawson, I.K. Mellinghoff, P.S. Mischel, M. Phelps, L.F. Parada, X. Liu, M.V. Sofroniew, F.C. Eilber, H. Wu, PTEN dosage is essential for neurofibroma development and malignant transformation. Proc. Natl. Acad. Sci. U. S. A. 106, 19479–19484 (2009). https://doi.org/10.1073/pnas.0910398106
J. Shan, Z. Wang, Q. Mo, J. Long, Y. Fan, L. Cheng, T. Zhang, X. Liu, X. Wang, Ribonucleotide reductase M2 subunit silencing suppresses tumorigenesis in pancreatic cancer via inactivation of PI3K/AKT/mTOR pathway. Pancreatology 22, 401–413 (2022). https://doi.org/10.1016/j.pan.2022.03.002
S. Zhang, L. Yan, C. Cui, Z. Wang, J. Wu, A. Lv, M. Zhao, B. Dong, W. Zhang, X. Guan, X. Tian, C. Hao, Downregulation of RRM2 Attenuates Retroperitoneal Liposarcoma Progression via the Akt/mTOR/4EBP1 Pathway: Clinical, Biological, and Therapeutic Significance. Onco. Targets Ther. 13, 6523–6537 (2020). https://doi.org/10.2147/OTT.S246613
Y.Z. Mazzu, J. Armenia, G. Chakraborty, Y. Yoshikawa, S.A.A. Coggins, S. Nandakumar, T.A. Gerke et al., A novel mechanism driving poor-prognosis prostate cancer: overexpression of the DNA Repair Gene, Ribonucleotide Reductase Small Subunit M2 (RRM2). Clin. Cancer Res. 25, 4480–4492 (2019). https://doi.org/10.1158/1078-0432.CCR-18-4046
Y. Aye, M. Li, M.J.C. Long, R.S. Weiss, Ribonucleotide reductase and cancer: biological mechanisms and targeted therapies. Oncogene 34, 2011–2021 (2015). https://doi.org/10.1038/onc.2014.155
J. Shao, X. Liu, L. Zhu, Y. Yen, Targeting ribonucleotide reductase for cancer therapy. Expert Opin. Ther. Targets 17, 1423–1437 (2013). https://doi.org/10.1517/14728222.2013.840293
Y. Zhan, L. Jiang, X. Jin, S. Ying, Z. Wu, L. Wang, W. Yu, J. Tong, L. Zhang, Y. Lou, Y. Qiu, Inhibiting RRM2 to enhance the anticancer activity of chemotherapy. Biomed. Pharmacother. 133, 110996 (2021). https://doi.org/10.1016/j.biopha.2020.110996
S. Ohmura, A. Marchetto, M.F. Orth, J. Li, S. Jabar, A. Ranft, E. Vinca, K. Ceranski, M.J. Carreño-Gonzalez et al., Translational evidence for RRM2 as a prognostic biomarker and therapeutic target in Ewing sarcoma. Mol. Cancer 20, 97 (2021). https://doi.org/10.1186/s12943-021-01393-9
L. Dai, Z. Lin, J. Qiao, Y. Chen, E.K. Flemington, Z. Qin, Ribonucleotide reductase represents a novel therapeutic target in primary effusion lymphoma. Oncogene 36, 5068–5074 (2017). https://doi.org/10.1038/onc.2017.122
Y. Yen, K. Margolin, J. Doroshow, M. Fishman, B. Johnson, C. Clairmont, D. Sullivan, M. Sznol, A phase I trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone in combination with gemcitabine for patients with advanced cancer. Cancer Chemother Pharmacol 54, 331–342 (2004)
C.A. Kunos, T. Radivoyevitch, S. Waggoner, R. Debernardo, K. Zanotti, K. Resnick, N. Fusco, R. Adams, R. Redline, P. Faulhaber, A. Dowlati, Radiochemotherapy plus 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in advanced-stage cervical and vaginal cancers. Gynecol. Oncol. 130, 75–80 (2013). https://doi.org/10.1016/j.ygyno.2013.04.019
Acknowledgements
We thank Prof. Vincent Keng and Prof. Jilong Yang for providing all the MPNST cell lines.
Funding
This work was supported by grants from National Natural Science Foundation of China (82202470; 82102344; 82172228); Shanghai Rising Star Program supported by Science and Technology Commission of Shanghai Municipality (20QA1405600); Natural Science Foundation of Shanghai (22ZR1422300); Innovative research team of high-level local universities in Shanghai (SHSMU-ZDCX20210400); Clinical Research Plan of SHDC (SHDC2020CR1019B); Shanghai Clinical Research Center of Plastic and Reconstructive Surgery supported by Science and Technology Commission of Shanghai Municipality (Grant No. 22MC1940300).
Author information
Authors and Affiliations
Contributions
Conception and design: M.-H.C, Z.W., and Q.Y.; Acquisition of data: M.-H.C, R.A., Y.L., H.L. Y.G., W.W., C.W., Z.G., and M.L.;Analysis and interpretation: M.-H.C, R.A., and Y.L;Writing, re-view of the manuscript: M.-H.C, Z.W., and Q.L.; All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval statement
The study involving human participants was reviewed and approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. Informed consent was obtained from patients under institutional review board protocols. The animal study was reviewed and approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine.
Competing interest statement
The authors declare no conflicts of interest associated with this publication.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chung, MH., Aimaier, R., Yu, Q. et al. RRM2 as a novel prognostic and therapeutic target of NF1-associated MPNST. Cell Oncol. 46, 1399–1413 (2023). https://doi.org/10.1007/s13402-023-00819-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-023-00819-4