Abstract
Purpose
CFTR mutations not only cause cystic fibrosis, but also increase the risk of colorectal cancer. A putative role of CFTR in colorectal cancer patients without cystic fibrosis has so far, however, not been investigated. RAC3 is a nuclear receptor coactivator that has been found to be overexpressed in several human tumors, and to be required for maintaining cancer stemness. Here, we investigated the functional relationship between CFTR and RAC3 for maintaining cancer stemness in human colorectal cancer.
Methods
Cancer stemness was investigated by analysing the expression of stem cell markers, clonogenic growth and selective retention of fluorochrome, using stable transfection of shCFTR or shRAC3 in HCT116 colorectal cancer cells. In addition, we performed pathway enrichment and network analyses in both primary human colorectal cancer samples (TCGA, Xena platform) and Caco-2 colorectal cancer cells including (1) CD133+ or CD133- side populations and (2) CFTRwt or CFTRmut cells (ConsensusPathDB, STRING, Cytoscape, GeneMANIA).
Results
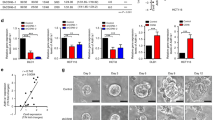
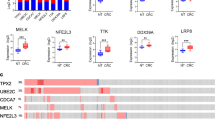
We found that the CD133+ side population expresses higher levels of RAC3 and CFTR than the CD133- side population. RAC3 overexpression increased CFTR expression, whereas CFTR downregulation inhibited the cancer stem phenotype. CFTR mRNA levels were found to be increased in colorectal cancer samples from patients without cystic fibrosis compared to those with CFTR mutations, and this correlated with an increased expression of RAC3. The expression pattern of a gene set involved in inflammatory response and nuclear receptor modulation in CD133+ Caco-2 cells was found to be shared with that in CFTRwt Caco-2 cells. These genes may contribute to colorectal cancer development.
Conclusions
CFTR may play a non-tumor suppressor role in colorectal cancer development and maintenance involving enhancement of the expression of a set of genes related to cancer stemness and development in patients without CFTR mutations.







Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- ABC transporters:
-
ATP-Binding Cassette transporters
- ATP:
-
Adenosine Triphosphate
- BSA:
-
Bovine Serum Albumin
- CFTR:
-
Cystic Fibrosis Transmembrane Conductance Regulator
- EGF:
-
Epidermal Growth Factor
- MDR:
-
Multiple Drug Resistance
- NF-ҡB:
-
Nuclear Factor Kappa B
- OCT4:
-
Octamer-Binding Transcription Factor 4
- PBS:
-
Phosphate Buffered Saline
- PPI:
-
Protein-Protein Interaction
- shCFTR:
-
Short Hairpin for CFTR
- shRAC3:
-
Short Hairpin for RAC3
- TNF:
-
Tumor Necrosis Factor
References
K.J. Anderson, R.T. Cormier, P.M. Scott, Role of ion channels in gastrointestinal cancer. World J. Gastroenterol. 25, 5732–5772 (2019). https://doi.org/10.3748/wjg.v25.i38.5732
A.G. Palma, L. Galizia, B.A. Kotsias, G.I. Marino, CFTR channel in oocytes from Xenopus laevis and its regulation by xShroom1 protein. Eur. J. Physiol. 468, 871–880 (2016). https://doi.org/10.1007/s00424-016-1800-2
D.G. Tang, Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 22, 457–472 (2012). doi:https://doi.org/10.1038/cr.2012.13
P. Scott, K. Anderson, M. Singhania, R. Cormier, Cystic fibrosis, CFTR, and colorectal cancer. Int. J. Mol. Sci. 21, 2891 (2020). https://doi.org/10.3390/ijms21082891
B.L. Than, J.F. Linnekamp, T.K. Starr, D.A. Largaespada, A. Rod, Y. Zhang, V. Bruner, J. Abrahante, A. Schumann, T. Luczak, J. Walter, A. Niemczyk, M.G. O’Sullivan, J.P. Medema, R.J. Fijneman, G.A. Meijer, E. Van den Broek, C.A. Hodges, P.M. Scott, L. Vermeulen, R.T. Cormier, CFTR is a tumor suppressor gene in murine and human intestinal cancer. Oncogene 35, 4179–4187 (2016). https://doi.org/10.1038/onc.2015.483
B.B. Tysnes, R. Bjerkvig, Cancer initiation and progression: involvement of stem cells and the microenvironment. Biochim. Biophys. Acta 1775, 283–297 (2007). doi:https://doi.org/10.1016/j.bbcan.2007.01.001
C.T. Jordan, M.L. Guzman, M. Noble, Cancer stem cells. N. Engl. J. Med. 355, 1253–1261 (2006)
C. Sakakura, A. Hagiwara, R. Yasuoka, Y. Fujita, M. Nakanishi, K. Masuda, A. Kimura, Y. Nakamura, J. Inazawa, T. Abe, H. Yamagishi, Amplification and over-expression of the AIB1 nuclear receptor co-activator gene in primary gastric cancers. Int. J. Cancer 89, 217–223 (2000)
S. Anzick, J. Kononen, R. Walker, D. Azorsa, M. Tanner, X. Guan, G. Sauter, O. Kallioniemi, J. Trent, P. Meltzer, AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277, 965–968 (1997)
R.T. Henke, B.R. Haddad, S.E. Kim, J.D. Rone, A. Mani, J.M. Jessup, A. Wellstein, A. Maitra, A.T. Riegel, Overexpression of the nuclear receptor coactivator AIB1 (SRC-3) during progression of pancreatic adenocarcinoma. Clin. Cancer Res. 10, 6134–6142 (2004)
H.J. Zhou, J. Yan, W. Luo, G. Ayala, S.H. Lin, H. Erdem, M. Ittmann, S.Y. Tsai, M.J. Tsai, SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 65, 7976–7983 (2005)
L.C. Panelo, M.S. Machado, M.F. Rubio, F. Jaworski, C.V. Alvarado, L.A. Paz, A.J. Urtreger, E. Vazquez, M.A. Costas, High RAC3 expression levels are required for induction and maintaining of cancer cell stemness. Oncotarget 9, 5848–5860 (2018). doi:https://doi.org/10.18632/oncotarget.23635
M.S. Machado, F.D. Rosa, M.C. Lira, A.J. Urtreger, M.F. Rubio, M.A. Costas, The inflammatory cytokine TNF contributes with RAC3-induced malignant transformation. EXCLI J. 17, 1030–1042 (2018). https://doi.org/10.17179/excli2018-1759
S. Werbajh, I. Nojek, R. Lanz, M.A. Costas, RAC-3 is a NF-kB coactivator. FEBS Lett. 485, 195–199 (2000)
G.P. Colo, R.R. Rosato, S. Grant, M.A. Costas, RAC3 down-regulation sensitizes human chronic myeloid leukemia cells to TRAIL-induced apoptosis. FEBS Lett. 581, 5075–5081 (2007)
G.P. Colo, M.F. Rubio, I.M. Nojek, S.E. Werbajh, P.C. Echeverria, C.V. Alvarado, V.E. Nahmod, M.D. Galigniana, M.A. Costas, The p160 nuclear receptor co-activator RAC3 exerts an anti-apoptotic role through a cytoplasmatic action. Oncogene 27, 2430–2444 (2008)
P.N. Fernandez Larrosa, M. Ruiz Grecco, D. Mengual Gomez, C.V. Alvarado, L.C. Panelo, M.F. Rubio, D.F. Alonso, D.E. Gomez and M.A. Costas. RAC3 more than a nuclear receptor coactivator: a key inhibitor of senescence that is downregulated in aging. Cell Death Dis 6, e1902 (2015) https://doi.org/10.1038/cddis.2015.218
M.F. Rubio, M.C. Lira, F.D. Rosa, A.D. Sambresqui, Salazar Guemes and M.A. Costas. RAC3 influences the chemoresistance of colon cancer cells through autophagy and apoptosis inhibition. Cancer Cell Int. 17, 111 (2017). https://doi.org/10.1186/s12935-017-0483-x
G. Ma, Y. Ren, K. Wang, J. He, SRC-3 has a role in cancer other than as a nuclear receptor coactivator. Int. J. Biol. Sci. 7, 664–672 (2011)
C.V. Alvarado, M.F. Rubio, P.N. Fernandez Larrosa, L. Panelo, P.J. Azurmendi, M. Ruiz Grecco, G.A. Martínez-Noël, M.A. Costas, The levels of RAC3 expression are up regulated by TNF in the inflammatory response. FEBS Open Bio 4, 450–457 (2014). https://doi.org/10.1016/j.fob.2014.04.009
N. Ameen, J. Alexis, P. Salas, Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem. Cell Biol. 114, 69–75 (2000). doi:https://doi.org/10.1007/s004180000164
A.M. Strubberg, J. Liu, N.M. Walker, C.D. Stefanski, R.J. MacLeod, S.T. Magness, L.L. Clarke, Cftr modulates Wnt/beta-catenin signaling and stem cell proliferation in murine intestine. Cell. Mol. Gastroenterol. Hepatol. 5, 253–271 (2018). https://doi.org/10.1016/j.jcmgh.2017.11.013
G.M. Seigel, L.M. Campbell, High-throughput microtiter assay for Hoechst 33342 dye uptake. Cytotechnology 45, 155–160 (2004). doi:https://doi.org/10.1007/s10616-004-7256-9
S. Hao, E.A. Roesch, A. Perez, R.L. Weiner, L.C. Henderson, L. Cummings, P. Consiglio, J. Pajka, A. Eisenberg, L. Yeh, C.U. Cotton, M.L. Drumm, Inactivation of CFTR by CRISPR/Cas9 alters transcriptional regulation of inflammatory pathways and other networks. J. Cyst. Fibros. 19, 34–39 (2020). https://doi.org/10.1016/j.jcf.2019.05.003
R. Herwig, C. Hardt, M. Lienhard, A. Kamburov, Analyzing and interpreting genome data at the network level with ConsensusPathDB. Nat. Protoc. 11, 1889–1907 (2016). https://doi.org/10.1038/nprot.2016.117
M.J. Goldman, B. Craft, M. Hastie, K. Repecka, F. McDade, A. Kamath, A. Banerjee, Y. Luo, D. Rogers, A.N. Brooks, J. Zhu, D. Haussler, Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38, 675–678 (2020). https://doi.org/10.1038/s41587-020-0546-8
X. Yu, Y. Lin, X. Yan, Q. Tian, L. Li, E.H. Lin, CD133, Stem cells, and cancer stem cells: Myth or reality? Curr. Colorect. Cancer Rep. 7, 253–259 (2011). https://doi.org/10.1007/s11888-011-0106-1
K. Liu, X. Zhang, J.T. Zhang, L.L. Tsang, X. Jiang, H.C. Chan, Defective CFTR- beta-catenin interaction promotes NF-kappaB nuclear translocation and intestinal inflammation in cystic fibrosis. Oncotarget 7, 64030–64042 (2016). doi:https://doi.org/10.18632/oncotarget.11747
L. Zeng, Q. Xiao, M. Chen, A. Margariti, D. Martin, A. Ivetic, H. Xu, J. Mason, W. Wang, G. Cockerill, K. Mori, J.Y. Li, S. Chien, Y. Hu, Q. Xu, Vascular endothelial cell growth-activated XBP1 splicing in endothelial cells is crucial for angiogenesis. Circulation 127, 1712–1722 (2013). doi:https://doi.org/10.1161/CIRCULATIONAHA.112.001337
P. Li, J. Singh, Y. Sun, X. Ma, P. Yuan, CFTR constrains the differentiation from mouse embryonic stem cells to intestine lineage cells. Biochem. Biophys. Res. Commun. 510, 322–328 (2019). https://doi.org/10.1016/j.bbrc.2019.01.100
R.A. Padua, N. Warren, D. Grimshaw, M. Smith, C. Lewis, J. Whittaker, P. Laidler, P. Wright, A. Douglas-Jones, P. Fenaux, A. Sharma, K. Horgan and R. West. The cystic fibrosis delta F508 gene mutation and cancer. Hum. Mut. 10, 45–48 (1997). https://doi.org/10.1002/(SICI)1098-1004(1997)10:1<45::AID-HUMU6>3.0.CO;2-L
R.L. Jakab, A.M. Collaco, N.A. Ameen, Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am. J. Physiol. 300, G82–G98 (2011). https://doi.org/10.1152/ajpgi.00245.2010
N. Barker, J.H. van Es, J. Kuipers, P. Kujala, M. van den Born, M. Cozijnsen, A. Haegebarth, J. Korving, H. Begthel, P.J. Peters, H. Clevers, Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007). doi:https://doi.org/10.1038/nature06196
U. Bedi, V.K. Mishra, D. Wasilewski, C. Scheel, S.A. Johnsen, Epigenetic plasticity: a central regulator of epithelial-to-mesenchymal transition in cancer. Oncotarget 5, 2016–2029 (2014). doi:https://doi.org/10.18632/oncotarget.1875
X. Yang, T. Yan, Y. Gong, X. Liu, H. Sun, W. Xu, C. Wang, D. Naren, Y. Zheng, High CFTR expression in Philadelphia chromosome-positive acute leukemia protects and maintains continuous activation of BCR-ABL and related signaling pathways in combination with PP2A. Oncotarget 8, 24437–24448 (2017). https://doi.org/10.18632/oncotarget.15510
G.I. Marino, B.A. Kotsias, Cystic fibrosis transmembrane regulator (CFTR) in human trophoblast BeWo cells and its relation to cell migration. Placenta 35, 92–98 (2014). doi:https://doi.org/10.1016/j.placenta.2013.12.004
A.M. Crane, P. Kramer, J.H. Bui, W.J. Chung, X.S. Li, M.L. Gonzalez-Garay, F. Hawkins, W. Liao, D. Mora, S. Choi, J. Wang, H.C. Sun, D.E. Paschon, D.Y. Guschin, P.D. Gregory, D.N. Kotton, M.C. Holmes, E.J. Sorscher, B.R. Davis, Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Rep. 4, 569–577 (2015). https://doi.org/10.1016/j.stemcr.2015.02.005
J.T. Zhang, Y. Wang, J.J. Chen, X.H. Zhang, J.D. Dong, L.L. Tsang, X.R. Huang, Z. Cai, H.Y. Lan, X.H. Jiang, H.C. Chan, Defective CFTR leads to aberrant beta-catenin activation and kidney fibrosis. Sci. Rep. 7, 5233 (2017). https://doi.org/10.1038/s41598-017-05435-5
Z. Li, CD133: a stem cell biomarker and beyond. Exp. Hematol. Oncol. 2, 17 (2013). https://doi.org/10.1186/2162-3619-2-17
J.M. Loo, A. Scherl, A. Nguyen, F.Y. Man, E. Weinberg, Z. Zeng, L. Saltz, P.B. Paty, S.F. Tavazoie, Extracellular metabolic energetics can promote cancer progression. Cell 160, 393–406 (2015). https://doi.org/10.1016/j.cell.2014.12.018
Funding
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas [PIP 11220150100118CO] and Agencia Nacional de Promoción Científica y Tecnológica [PICT 1424, PBID 2014].
Author information
Authors and Affiliations
Contributions
Alejandra Graciela Palma: methodology, investigation, visualization, formal analysis; Mileni Soares Machado: methodology, investigation, visualization; María Cecilia Lira: investigation, formal analysis; Francisco Rosa: investigation, formal analysis; María Fernanda Rubio: Review and editing, visualization; Gabriela Marino: formal analysis, visualization; Basilio Aristidis Kotsias: conceptualization, writing, review and editing; Mónica Alejandra Costas: supervision, conceptualization, funding acquisition, project administration, original draft writing, formal analysis, visualization.
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
We wish to confirm that there are no known conflicts of interest associated with this publication and that there has been no significant financial support for this work that could have influenced its outcome.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 948 kb)
Rights and permissions
About this article
Cite this article
Palma, A.G., Soares Machado, M., Lira, M.C. et al. Functional relationship between CFTR and RAC3 expression for maintaining cancer cell stemness in human colorectal cancer. Cell Oncol. 44, 627–641 (2021). https://doi.org/10.1007/s13402-021-00589-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-021-00589-x