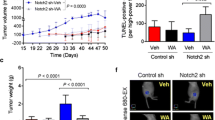
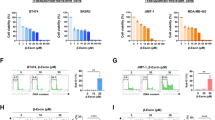
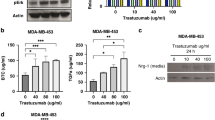
Abstract
Purpose
Isothiocyanates elicit anticancer effects by targeting cancer stem cells (CSCs). Here, we tested the antitumor activity of phenethyl-isothiocyanate (PEITC), either alone or in combination with trastuzumab, in HER2-positive tumor models.
Methods
We assessed the in vitro anticancer activity of PEITC, alone or combined with trastuzumab, in HER2-positive BT474, SKBR3, HCC1954 and SKOV3 cancer cells by measuring their sphere forming efficiency (SFE). The expression of the human/rodent CSC biomarkers aldehyde-dehydrogenase (ALDH) and CD29High/CD24+/Sca1Low was evaluated by cytofluorimetric analysis. The expression of wild type HER2 (WTHER2), its splice variant d16HER2 and NOTCH was analysed by quantitative RT-PCR and Western blotting. The in vivo activity of PEITC and trastuzumab was evaluated in mice orthotopically implanted with MI6 tumor cells transgenic for the human d16HER2 splice isoform. Magnetic resonance imaging/spectroscopy and immunohistochemistry were used to assess morpho-functional and metabolic profiles of treated versus untreated mice.
Results
We found that PEITC significantly impaired the SFE of HER2-positive human cancer cells by decreasing their ALDH-positive compartments. The anti-CSC activity of PEITC was demonstrated by a reduced expression/activation of established cancer-stemness biomarkers. Similar results were obtained with MI6 cells, where PEITC, alone or in combination with trastuzumab, significantly inhibited their SFE. We also found that PEITC hampered the in vivo growth of MI6 nodules by inducing hemorrhagic and necrotic intra-tumor areas and, in combination with trastuzumab, by significantly reducing spontaneous tumor development in d16HER2 transgenic mice.
Conclusions
Our results indicate that PEITC targets HER2-positive CSCs and that its combination with trastuzumab may pave the way for a novel therapeutic strategy for HER2-positive tumors.




Similar content being viewed by others
References
S. Parakh, H.K. Gan, A.C. Parslow, I.J.G. Burvenich, A.W. Burgess, A.M. Scott, Evolution of anti-HER2 therapies for cancer treatment. Cancer Treat. Rev. 59, 1–21 (2017). https://doi.org/10.1016/j.ctrv.2017.06.005
B.N. Rexer, C.L. Arteaga, Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: Mechanisms and clinical implications. Crit. Rev. Oncog. 17, 1–16 (2012)
S. Loibl, L. Gianni, HER2-positive breast cancer. Lancet 389, 2415–2429 (2017). https://doi.org/10.1016/S0140-6736(16)32417-5
P. Samadi, S. Saki, F.K. Dermani, M. Pourjafar, M. Saidijam, Emerging ways to treat breast cancer: Will promises be met? Cell. Oncol. (Dordr). 41(605–621), 605–621 (2018). https://doi.org/10.1007/s13402-018-0409-1
T. Triulzi, G.V. Bianchi, E. Tagliabue, Predictive biomarkers in the treatment of HER2-positive breast cancer: An ongoing challenge. Future Oncol. 12, 1413–1428 (2016). https://doi.org/10.2217/fon-2015-0025
C.T. Jordan, M.L. Guzman, M. Noble, Cancer stem cells. N. Engl. J. Med. 355, 1253–1261 (2006). https://doi.org/10.1056/NEJMra061808
H. Korkaya, S. Liu, M.S. Wicha, Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J. Clin. Invest. 121, 3804–3809 (2011). https://doi.org/10.1172/JCI57099
H. Korkaya, M.S. Wicha, Breast cancer stem cells: we've got them surrounded. Clin. Cancer Res. 19, 511–513 (2013). https://doi.org/10.1158/1078-0432.CCR-12-3450
R. Castro-Oropeza, J. Melendez-Zajgla, V. Maldonado, K. Vazquez-Santillan, The emerging role of lncRNAs in the regulation of cancer stem cells. Cell. Oncol. (Dordr) 41(585–603), 585–603 (2018). https://doi.org/10.1007/s13402-018-0406-4
H. Korkaya, M.S. Wicha, HER2 and breast cancer stem cells: more than meets the eye. Cancer Res. 73, 3489–3493 (2013). https://doi.org/10.1158/0008-5472.CAN-13-0260
S. Annett, T. Robson, Targeting cancer stem cells in the clinic: Current status and perspectives. Pharmacol. Ther. 187, 13–30 (2018). https://doi.org/10.1016/j.pharmthera.2018.02.001
S.G. Pohl, N. Brook, M. Agostino, F. Arfuso, A.P. Kumar, A. Dharmarajan, Wnt signaling in triple-negative breast cancer. Oncogenesis 6, e310 (2017). https://doi.org/10.1038/oncsis.2017.14
N. Shafee, C.R. Smith, S. Wei, Y. Kim, G.B. Mills, G.N. Hortobagyi, E.J. Stanbridge, E.Y. Lee, Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 68, 3243–3250 (2008). https://doi.org/10.1158/0008-5472.CAN-07-5480
D. Hambardzumyan, M. Squatrito, E.C. Holland, Radiation resistance and stem-like cells in brain tumors. Cancer Cell 10, 454–456 (2006). https://doi.org/10.1016/j.ccr.2006.11.008
L. Castagnoli, V. Cancila, S.L. Romero-Cordoba, S. Faraci, G. Talarico, B. Belmonte, M.V. Iorio, T. Volpari, C. Chiodoni, A. Hidalgo-Miranda, E. Tagliabue, C. Tripodo, S. Sangaletti, M. Di Nicola, S.M. Pupa, WNT signaling modulates PD-L1 expression in the stem cell compartment of triple-negative breast cancer. Oncogene 38, 4047–4060 (2019). https://doi.org/10.1038/s41388-019-0700-2
H. Korkaya, A. Paulson, F. Iovino, M.S. Wicha, HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 27, 6120–6130 (2008). https://doi.org/10.1038/onc.2008.207
A. Magnifico, L. Albano, S. Campaner, D. Delia, F. Castiglioni, P. Gasparini, G. Sozzi, E. Fontanella, S. Ménard, E. Tagliabue, Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to Trastuzumab. Clin. Cancer Res. 15, 2010–2021 (2009). https://doi.org/10.1158/1078-0432.CCR-08-1327
G. Farnie, R.L. Johnson, K.E. Williams, R.B. Clarke, N.J. Bundred, Lapatinib inhibits stem/progenitor proliferation in preclinical in vitro models of ductal carcinoma in situ (DCIS). Cell Cycle 13, 418–425 (2014). https://doi.org/10.4161/cc.27201
L. Castagnoli, G.C. Ghedini, A. Koschorke, T. Triulzi, M. Dugo, P. Gasparini, P. Casalini, A. Palladini, M. Iezzi, A. Lamolinara, P.L. Lollini, P. Nanni, C. Chiodoni, E. Tagliabue, S.M. Pupa, Pathobiological implications of the d16HER2 splice variant for stemness and aggressiveness of HER2-positive breast cancer. Oncogene 36, 1721–1732 (2017). https://doi.org/10.1038/onc.2016.338
K.Y. Kwong, M.C. Hung, A novel splice variant of HER2 with increased transformation activity. Mol. Carcinog. 23, 62–68 (1998)
F. Castiglioni, E. Tagliabue, M. Campiglio, S.M. Pupa, A. Balsari, S. Ménard, Role of exon-16-deleted HER2 in breast carcinomas. Endocr. Relat. Cancer 13, 221–232 (2006). https://doi.org/10.1677/ERC.1.01047
D. Mitra, M.J. Brumlik, S.U. Okamgba, Y. Zhu, T.T. Duplessis, J.G. Parvani, S.M. Lesko, E. Brogi, F.E. Jones, An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol. Cancer Ther. 8, 2152–2162 (2009). https://doi.org/10.1158/1535-7163.MCT-09-0295
C.C. Volpi, F. Pietrantonio, A. Gloghini, G. Fuca, S. Giordano, S. Corso, G. Pruneri, M. Antista, C. Cremolini, E. Fasano, S. Saggio, S. Faraci, M. Di Bartolomeo, F. De Braud, M. Di Nicola, E. Tagliabue, S.M. Pupa, L. Castagnoli, The landscape of d16HER2 splice variant expression across HER2-positive cancers. Sci. Rep. 9, 3545 (2019). https://doi.org/10.1038/s41598-019-40310-5
C. Marchini, F. Gabrielli, M. Iezzi, S. Zanobi, M. Montani, L. Pietrella, C. Kalogris, A. Rossini, V. Ciravolo, L. Castagnoli, E. Tagliabue, S.M. Pupa, P. Musiani, P. Monaci, S. Ménard, A. Amici, The human splice variant delta16HER2 induces rapid tumor onset in a reporter transgenic mouse. PLoS One 6, e18727 (2011). https://doi.org/10.1371/journal.pone.0018727
L. Castagnoli, M. Iezzi, G.C. Ghedini, V. Ciravolo, G. Marzano, A. Lamolinara, R. Zappasodi, P. Gasparini, M. Campiglio, A. Amici, C. Chiodoni, A. Palladini, P. Lollini, T. Triulzi, S. Ménard, P. Nanni, E. Tagliabue, S.M. Pupa, Activated d16HER2 homodimers and Src kinase mediate optimal efficacy for trastuzumab. Cancer Res. 74, 6248–6259 (2014). https://doi.org/10.1158/0008-5472.CAN-14-0983
A. Alajati, N. Sausgruber, N. Aceto, S. Duss, S. Sarret, H. Voshol, D. Bonenfant, M. tires-Alj, Mammary tumor formation and metastasis evoked by a HER2 splice variant. Cancer Res. 73, 5320–5327 (2013). https://doi.org/10.1158/0008-5472.CAN-12-3186
L. Castagnoli, E. Iorio, M. Dugo, A. Koschorke, S. Faraci, R. Canese, P. Casalini, P. Nanni, C. Vernieri, M. Di Nicola, D. Morelli, E. Tagliabue, S.M. Pupa, Intra-tumor lactate levels reflect HER2 addiction status in HER2-positive breast cancer. J. Cell. Physiol. 234, 1768–1779 (2019). https://doi.org/10.1002/jcp.27049
M.R. Doherty, J.M. Smigiel, D.J. Junk, M.W. Jackson, Cancer stem cell plasticity drives therapeutic resistance. Cancers (Basel) 8 (2016). https://doi.org/10.3390/cancers8010008
P. Gupta, B. Kim, S.H. Kim, S.K. Srivastava, Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 58, 1685–1707 (2014). https://doi.org/10.1002/mnfr.201300684
Y. Li, T. Zhang, H. Korkaya, S. Liu, H.F. Lee, B. Newman, Y. Yu, S.G. Clouthier, S.J. Schwartz, M.S. Wicha, D. Sun, Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 16, 2580–2590 (2010). https://doi.org/10.1158/1078-0432.CCR-09-2937
D. Hanahan, R.A. Weinberg, Hallmarks of cancer: The next generation. Cel. 144, 646–674 (2011). https://doi.org/10.1016/j.cell.2011.02.013
P. Gupta, S.E. Wright, S.H. Kim, S.K. Srivastava, Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. Biophys. Acta 1846, 405–424 (2014). https://doi.org/10.1016/j.bbcan.2014.08.003
K.S. Satyan, N. Swamy, D.S. Dizon, R. Singh, C.O. Granai, L. Brard, Phenethyl isothiocyanate (PEITC) inhibits growth of ovarian cancer cells by inducing apoptosis: Role of caspase and MAPK activation. Gynecol. Oncol. 103, 261–270 (2006). https://doi.org/10.1016/j.ygyno.2006.03.002
P. Gupta, S.K. Srivastava, Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Med. 10, 80 (2012). https://doi.org/10.1186/1741-7015-10-80
G.C. Ghedini, V. Ciravolo, M. Tortoreto, S. Giuffre, F. Bianchi, M. Campiglio, M. Mortarino, M. Figini, A. Coliva, M.L. Carcangiu, M. Zambetti, T. Piazza, S. Ferrini, S. Menard, E. Tagliabue, S.M. Pupa, Shed HER2 extracellular domain in HER2-mediated tumor growth and in trastuzumab susceptibility. J. Cell. Physiol. 225, 256–265 (2010). https://doi.org/10.1002/jcp.22257
F.L. Shaw, H. Harrison, K. Spence, M.P. Ablett, B.M. Simoes, G. Farnie, R.B. Clarke, A detailed mammosphere assay protocol for the quantification of breast stem cell activity. J. Mammary Gland Biol. Neoplasia 17, 111–117 (2012). https://doi.org/10.1002/jcp.22257
F. Granata, E. Iorio, G. Carpinelli, M. Giannini, F. Podo, Phosphocholine and phosphoethanolamine during chick embryo myogenesis: A (31)P-NMR study. Biochim. Biophys. Acta 1483, 334–342 (2000)
M.E. Pisanu, A. Ricci, L. Paris, E. Surrentino, L. Liliac, M. Bagnoli, S. Canevari, D. Mezzanzanica, F. Podo, E. Iorio, R. Canese, Monitoring response to cytostatic cisplatin in a HER2(+) ovary cancer model by MRI and in vitro and in vivo MR spectroscopy. Br. J. Cancer 110, 625–635 (2014). https://doi.org/10.1038/bjc.2013.758
C. Ginestier, M.H. Hur, E. Charafe-Jauffret, F. Monville, J. Dutcher, M. Brown, J. Jacquemier, P. Viens, C.G. Kleer, S. Liu, A. Schott, D. Hayes, D. Birnbaum, M.S. Wicha, G. Dontu, ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1, 555–567 (2007). https://doi.org/10.1016/j.stem.2007.08.014
A.T. Baker, A. Zlobin, C. Osipo, Notch-EGFR/HER2 bidirectional crosstalk in breast Cancer. Front. Oncol. 4, 360 (2014). https://doi.org/10.3389/fonc.2014.00360
Z.H. Liu, X.M. Dai, B. Du, Hes1: A key role in stemness, metastasis and multidrug resistance. Cancer Biol. Ther. 16, 353–359 (2015). https://doi.org/10.1080/15384047.2015.1016662
H.C. Thoeny, B.D. Ross, Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J. Magn. Reson. Imaging 32, 2–16 (2010). https://doi.org/10.1002/jmri.22167
M. Shackleton, F. Vaillant, K.J. Simpson, J. Stingl, G.K. Smyth, M.L. sselin-Labat, L. Wu, G.J. Lindeman, J.E. Visvader, Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 (2006). https://doi.org/10.1038/nature04372
E. Iorio, M. Di Vito, F. Spadaro, C. Ramoni, E. Lococo, R. Carnevale, L. Lenti, R. Strom, F. Podo, Triacsin C inhibits the formation of 1H NMR-visible mobile lipids and lipid bodies in HuT 78 apoptotic cells. Biochim. Biophys. Acta 1634, 1–14 (2003)
M. Tiefenthaler, A. Amberger, N. Bacher, B.L. Hartmann, R. Margreiter, R. Kofler, G. Konwalinka, Increased lactate production follows loss of mitochondrial membrane potential during apoptosis of human leukaemia cells. Br. J. Haematol. 114, 574–580 (2001)
G. Xu, J. Shen, Y. Ou, X, M. Sasahara, X. Su, Cancer stem cells: The 'heartbeat' of gastric cancer. J. Gastroenterol. 48, 781–797 (2013). https://doi.org/10.1007/s00535-012-0712-y
S. Palmer, Diet, nutrition, and cancer. Prog. Food Nutr. Sci. 9, 283–341 (1985)
D.T. Verhoeven, R.A. Goldbohm, P.G.H. van Verhagen, P.A. Van den Brandt, Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol. Biomark. Prev. 5, 733–748 (1996)
J.V. Higdon, B. Delage, D.E. Williams, R.H. Dashwood, Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 55, 224–236 (2007). https://doi.org/10.1016/j.phrs.2007.01.009
C.B. Ambrosone, S.E. McCann, J.L. Freudenheim, J.R. Marshall, Y. Zhang, P.G. Shields, Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J. Nutr. 134, 1134–1138 (2004)
D.A. Boggs, J.R. Palmer, L.A. Wise, D. Spiegelman, M.J. Stampfer, L.L. ms-Campbell, L. Rosenberg, Fruit and vegetable intake in relation to risk of breast cancer in the Black Women's Health Study. Am. J. Epidemiol. 172, 1268–1279 (2010). https://doi.org/10.1093/aje/kwq293
Y. Chen, Y. Li, X.Q. Wang, Y. Meng, Q. Zhang, J.Y. Zhu, J.Q. Chen, W.S. Cao, X.Q. Wang, C.F. Xie, X.T. Li, S.S. Geng, J.S. Wu, C.Y. Zhong, H.Y. Han, Phenethyl isothiocyanate inhibits colorectal cancer stem cells by suppressing Wnt/beta-catenin pathway. Phytother. Res. 32, 2447–2455 (2018). https://doi.org/10.1002/ptr.6183
G. Dontu, K.W. Jackson, E. McNicholas, M.J. Kawamura, W.M. Abdallah, M.S. Wicha, Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 6, R605–R615 (2004)
A. Marusyk, K. Polyak, Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta 1805, 105–117 (2010). https://doi.org/10.1016/j.bbcan.2009.11.002
J.P. Burnett, G. Lim, Y. Li, R.B. Shah, R. Lim, H.J. Paholak, S.P. McDermott, L. Sun, Y. Tsume, S. Bai, M.S. Wicha, D. Sun, T. Zhang, Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 394, 52–64 (2017). https://doi.org/10.1016/j.canlet.2017.02.023
G. Kallifatidis, V. Rausch, B. Baumann, A. Apel, B.M. Beckermann, A. Groth, J. Mattern, Z. Li, A. Kolb, G. Moldenhauer, P. Altevogt, T. Wirth, J. Werner, P. Schemmer, M.W. Buchler, A.V. Salnikov, I. Herr, Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut 58, 949–963 (2009). https://doi.org/10.1136/gut.2008.149039
Arpino, G., Ferrero, J. M., de la Haba-Rodriguez J., Easton, V., Schuhmacher, C., Restuccia, E. et al. Primary analysis of PERTAIN: a randomized, two-arm, open-label, multicenter phase II trial assessing the efficacy and safety of pertuzumab given in combination with trastuzumab plus an aromatase inhibitor in first-line patients with HER2-positive and hormone receptor-positive metastatic or locally advanced breast cancer. San Antonio Breast Cancer Symposium; December 6–10, 2016 San Antonio, Texas. 2017
S.R.D. Johnston, R. Hegg, S.A. Im, I.H. Park, O. Burdaeva, G. Kurteva, M.F. Press, S. Tjulandin, H. Iwata, S.D. Simon, S. Kenny, S. Sarp, M.A. Izquierdo, L.S. Williams, W.J. Gradishar, Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with Lapatinib plus Trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-Positive, hormone receptor-positive metastatic Breast Cancer: ALTERNATIVE. J. Clin. Oncol. 36, 741–748 (2018). https://doi.org/10.1200/JCO.2017.74.7824
Acknowledgments
The authors thank Mrs. C. Ghirelli for technical assistance, the Flow Cytometry and Cell Sorting Facility for their support in the cytofluorimetric analyses, Dr. P. Casalini for her support in the ImageJ analyses, Mrs. L. Mameli, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, for secretarial assistance and Dr. M. Borghi, Istituto Superiore di Sanità, Rome, for her contribution to the in vivo MRI study. This work was funded by grants from the Associazione Italiana Ricerca Cancro (AIRC; call 2010) 10352 to S.M. Pupa and Ministero Italiano della Salute RF-2009-1532281 to S.M. Pupa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
ESM 2
(DOCX 12 kb)
Supplementary Figure 1
Susceptibility of human HER2-positive BC cell lines to PEITC treatment. Cell growth of BT474, SKBR3, HCC1954, MDAMB231 and SKOV3 cell lines cultured under 2D conditions evaluated by SRB assay. MDAMB231 cell line was used as positive control for in vitro PEITC activity. Cells (3,000/well) were cultured and treated per 24, 48 and 72 h in presence of increasing concentrations of PEITC diluted in DMSO. (PDF 26 kb)
Supplementary Figure 2
Representative plots of ALDH-positive cells in BT474, SKBR3, HCC1954 and SKOV3 targets upon treatment with PEITC (12 μM) for 72 h. (PDF 231 kb)
Supplementary Figure 3
Susceptibility of MI6 cells to PEITC treatment. Cell growth of MI6 cell line cultured under 2D conditions evaluated by SRB assay. Cells (5,000/well) were cultured and treated per 24, 48 and 72 h in presence of increasing concentrations of PEITC diluted in DMSO. (PDF 102 kb)
Supplementary Figure 4
Representative images of morphology and size of MI6 mammospheres formed after 7 days in presence or not of T, PEITC and PEITC-T combination. Magnification 10X. (PDF 99 kb)
Supplementary Figure 5
Multiparametric FACS analysis of the CD29High/CD24+ SCA1Low stem cell subset in MI6 treated with DMSO, T, PEITC, and their combination (PEITC+T). (PDF 117 kb)
Rights and permissions
About this article
Cite this article
Koschorke, A., Faraci, S., Giani, D. et al. Phenethyl isothiocyanate hampers growth and progression of HER2-positive breast and ovarian carcinoma by targeting their stem cell compartment. Cell Oncol. 42, 815–828 (2019). https://doi.org/10.1007/s13402-019-00464-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-019-00464-w