Abstract
Purpose
Emerging evidence indicates that bromodomains comprise a conserved class of epigenome readers involved in cancer development and inflammation. Bromodomains are associated with epigenetic modifications of gene transcription through interactions with lysine residues of histone tails. Particularly, the bromodomain and extra-terminal domain (BET) family member BRD4 has been found to be involved in the control over oncogenes, including c-MYC, and in the maintenance of downstream inflammatory processes. The objective of this study was to evaluate the effect of pharmacologically displacing BRD4 in mucoepidermoid carcinoma (MEC) cells.
Methods
We assessed the presence of BRD4 levels in a panel of human MEC tissue samples in conjunction with histological grading and clinical information. In vitro studies were carried out using human MEC-derived cell lines. The BET inhibitor iBET762 was administered to MEC cells to assess the impact of disrupted BRD4 signaling on colony forming capacities and cell cycle status. The activation of cellular senescence induced by iBET762 was determined by immunohistochemical staining for p16ink4. Flow cytometry was used to identify populations of cancer stem cells in MEC-derived cell lines.
Results
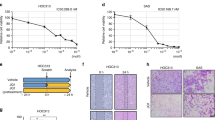
We found that primary human MECs and MEC-derived cell lines are endowed with high BRD4 expression levels compared to those in normal salivary glands. We also found that, by displacing BRD4 from chromatin using the BET inhibitor iBET762, MEC cells lose their colony forming capacities and undergo G1 cell cycle arrest and senescence. Finally, we found that targeted displacement of BRD4 from chromatin results in depletion of cancer stem cells from the overall MEC cell populations.
Conclusions
Our findings indicate that bromodomain-mediated gene regulation constitutes an epigenetic mechanism that is deregulated in MEC cells and that the use of BET inhibitors may serve as a feasible therapeutic strategy to manage MECs.





Similar content being viewed by others
References
P.A. Vargas, R. Gerhard, V.J. Araujo Filho, I.V. de Castro, Salivary gland tumors in a Brazilian population: A retrospective study of 124 cases. Rev. Hosp. Clin. Fac. Med. Sao Paulo 57, 271–276 (2002)
F.A. de Oliveira, E.C. Duarte, C.T. Taveira, A.A. Maximo, E.C. de Aquino, C. Alencar Rde, E.F. Vencio, Salivary gland tumor: a review of 599 cases in a Brazilian population. Head Neck Pathol. 3, 271–275 (2009)
F.P. Fonseca, V. Carvalho Mde, O.P. de Almeida, A.L. Rangel, M.C. Takizawa, A.G. Bueno, P.A. Vargas, Clinicopathologic analysis of 493 cases of salivary gland tumors in a southern Brazilian population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 114, 230–239 (2012)
E.S. Choi, S. Oh, B. Jang, H.J. Yu, J.A. Shin, N.P. Cho, I.H. Yang, D.H. Won, H.J. Kwon, S.D. Hong, S.D. Cho, Silymarin and its active component silibinin act as novel therapeutic alternatives for salivary gland cancer by targeting the ERK1/2-Bim signaling cascade. Cell Oncol. 40, 235–246 (2017)
N. Saghravanian, N. Ghazi, M. Saba, Clinicopathologic evaluation of salivary gland neoplasms: a 38-year retrospective study in Iran. Ann. Diagn. Pathol. 17, 522–525 (2013)
J.H. Mikesch, W. Hartmann, L. Angenendt, O. Huber, C. Schliemann, M.F. Arteaga, E. Wardelmann, C. Rudack, W.E. Berdel, M. Stenner and I. Grunewald, AAA+ ATPases Reptin and Pontin as potential diagnostic and prognostic biomarkers in salivary gland cancer - a short report. Cell Oncol. 41, 455–462 (2018)
L. Barnes, Universitäts-Spital Zurich. Dept. Pathologie., International Academy of Pathology., World Health Organization. and International Agency for Research on Cancer., Pathology and genetics of head and neck tumours, (IARC Press, Lyon, 2007)
M.R. Posner, T.J. Ervin, R.R. Weichselbaum, R.L. Fabian, D. Miller, Chemotherapy of advanced salivary gland neoplasms. Cancer 50, 2261–2264 (1982)
S. Grisanti, V. Amoroso, M. Buglione, A. Rosati, R. Gatta, C. Pizzocaro, V.D. Ferrari, G. Marini, Cetuximab in the treatment of metastatic mucoepidermoid carcinoma of the salivary glands: a case report and review of literature. J. Med. Case Rep. 2, 320 (2008)
T. Cerda, X.S. Sun, S. Vignot, P.Y. Marcy, B. Baujat, A.C. Baglin, A.M. Ali, S. Testelin, E. Reyt, F. Janot, J. Thariat, A rationale for chemoradiation (vs radiotherapy) in salivary gland cancers? On behalf of the REFCOR (French rare head and neck cancer network). Crit. Rev. Oncol. Hematol. 91, 142–158 (2014)
A. Coca-Pelaz, J.P. Rodrigo, A. Triantafyllou, J.L. Hunt, A. Rinaldo, P. Strojan, M. Haigentz Jr., W.M. Mendenhall, R.P. Takes, V. Vander Poorten, A. Ferlito, Salivary mucoepidermoid carcinoma revisited. Eur. Arch. Otorhinolaryngol. 272, 799–819 (2015)
R.M. Castilho, C.H. Squarize, L.O. Almeida, Epigenetic modifications and head and neck Cancer: Implications for tumor progression and resistance to therapy. Int J Mol Sci. 18 E15063 (2017)
M.S. Gilardini Montani, M. Granato, C. Santoni, P. Del Porto, N. Merendino, G. D'Orazi, A. Faggioni, M. Cirone, Histone deacetylase inhibitors VPA and TSA induce apoptosis and autophagy in pancreatic cancer cells. Cell Oncol. 40, 167–180 (2017)
M. Staberg, S.R. Michaelsen, R.D. Rasmussen, M. Villingshoj, H.S. Poulsen, P. Hamerlik, Inhibition of histone deacetylases sensitizes glioblastoma cells to lomustine. Cell Oncol. 40, 21–32 (2017)
M.F. Segura, B. Fontanals-Cirera, A. Gaziel-Sovran, M.V. Guijarro, D. Hanniford, G. Zhang, P. Gonzalez-Gomez, M. Morante, L. Jubierre, W. Zhang, F. Darvishian, M. Ohlmeyer, I. Osman, M.M. Zhou, E. Hernando, BRD4 sustains melanoma proliferation and represents a new target for epigenetic therapy. Cancer Res. 73, 6264–6276 (2013)
M. Perez-Salvia, M. Esteller, Bromodomain inhibitors and cancer therapy: From structures to applications. Epigenetics 12, 323–339 (2017)
C.A. French, I. Miyoshi, J.C. Aster, I. Kubonishi, T.G. Kroll, P. Dal Cin, S.O. Vargas, A.R. Perez-Atayde, J.A. Fletcher, BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19). Am. J. Pathol. 159, 1987–1992 (2001)
D. Houzelstein, S.L. Bullock, D.E. Lynch, E.F. Grigorieva, V.A. Wilson, R.S. Beddington, Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol. Cell. Biol. 22, 3794–3802 (2002)
K. Mochizuki, A. Nishiyama, M.K. Jang, A. Dey, A. Ghosh, T. Tamura, H. Natsume, H. Yao, K. Ozato, The bromodomain protein Brd4 stimulates G1 gene transcription and promotes progression to S phase. J. Biol. Chem. 283, 9040–9048 (2008)
V.P. Wagner, M.A. Martins, M.D. Martins, K.A. Warner, L.P. Webber, C.H. Squarize, J.E. Nor, R.M. Castilho, Overcoming adaptive resistance in mucoepidermoid carcinoma through inhibition of the IKK-beta/IkappaBalpha/NFkappaB axis. Oncotarget 7, 73032–73044 (2016)
N.A. Franken, H.M. Rodermond, J. Stap, J. Haveman, C. van Bree, Clonogenic assay of cells in vitro. Nat. Protoc. 1, 2315–2319 (2006)
M.D. Martins, Y. Jiao, L. Larsson, L.O. Almeida, C. Garaicoa-Pazmino, J.M. Le, C.H. Squarize, N. Inohara, W.V. Giannobile, R.M. Castilho, Epigenetic modifications of histones in periodontal disease. J. Dent. Res. 95, 215–222 (2016)
E. Nicodeme, K.L. Jeffrey, U. Schaefer, S. Beinke, S. Dewell, C.W. Chung, R. Chandwani, I. Marazzi, P. Wilson, H. Coste, J. White, J. Kirilovsky, C.M. Rice, J.M. Lora, R.K. Prinjha, K. Lee, A. Tarakhovsky, Suppression of inflammation by a synthetic histone mimic. Nature 468, 1119–1123 (2010)
O. Mirguet, R. Gosmini, J. Toum, C.A. Clement, M. Barnathan, J.M. Brusq, J.E. Mordaunt, R.M. Grimes, M. Crowe, O. Pineau, M. Ajakane, A. Daugan, P. Jeffrey, L. Cutler, A.C. Haynes, N.N. Smithers, C.W. Chung, P. Bamborough, I.J. Uings, A. Lewis, J. Witherington, N. Parr, R.K. Prinjha, E. Nicodeme, Discovery of epigenetic regulator I-BET762: Lead optimization to afford a clinical candidate inhibitor of the BET bromodomains. J. Med. Chem. 56, 7501–7515 (2013)
S. Wang, A.M. Pike, S.S. Lee, M.A. Strong, C.J. Connelly, C.W. Greider, BRD4 inhibitors block telomere elongation. Nucleic Acids Res. 45, 8403–8410 (2017)
L.O. Almeida, D.M. Guimaraes, M.D. Martins, M.A.T. Martins, K.A. Warner, J.E. Nor, R.M. Castilho, C.H. Squarize, Unlocking the chromatin of adenoid cystic carcinomas using HDAC inhibitors sensitize cancer stem cells to cisplatin and induces tumor senescence. Stem Cell Res. 21, 94–105 (2017)
A. Newbold, K.J. Falkenberg, H.M. Prince, R.W. Johnstone, How do tumor cells respond to HDAC inhibition? FEBS J. 283, 4032–4046 (2016)
D.M. Guimaraes, L.O. Almeida, M.D. Martins, K.A. Warner, A.R. Silva, P.A. Vargas, F.D. Nunes, C.H. Squarize, J.E. Nor, R.M. Castilho, Sensitizing mucoepidermoid carcinomas to chemotherapy by targeted disruption of cancer stem cells. Oncotarget 7, 42447–42460 (2016)
A. Adams, K. Warner, A.T. Pearson, Z. Zhang, H.S. Kim, D. Mochizuki, G. Basura, J. Helman, A. Mantesso, R.M. Castilho, M.S. Wicha, J.E. Nor, ALDH/CD44 identifies uniquely tumorigenic cancer stem cells in salivary gland mucoepidermoid carcinomas. Oncotarget 6, 26633–26650 (2015)
V.P. Wagner, M.D. Martins, M.A.T. Martins, L.O. Almeida, K.A. Warner, J.E. Nor, C.H. Squarize, R.M. Castilho, Targeting histone deacetylase and NFkappaB signaling as a novel therapy for Mucoepidermoid carcinomas. Sci. Rep. 8, 2065 (2018)
M. Granic, P. Suton, D. Mueller, I. Cvrljevic, I. Luksic, Prognostic factors in head and neck mucoepidermoid carcinoma: experience at a single institution based on 64 consecutive patients over a 28-year period. Int J Oral Maxillofac Surg. 47, 283-288 (2018)
A.C. Birkeland, S.K. Foltin, N.L. Michmerhuizen, R.C. Hoesli, A.J. Rosko, S. Byrd, M. Yanik, J.E. Nor, C.R. Bradford, M.E. Prince, T.E. Carey, J.B. McHugh, M.E. Spector, J.C. Brenner, Correlation of Crtc1/3-Maml2 fusion status, grade and survival in mucoepidermoid carcinoma. Oral Oncol. 68, 5–8 (2017)
B.N. Devaiah, C. Case-Borden, A. Gegonne, C.H. Hsu, Q. Chen, D. Meerzaman, A. Dey, K. Ozato, D.S. Singer, BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat. Struct. Mol. Biol. 23, 540–548 (2016)
X. Wu, D. Liu, X. Gao, F. Xie, D. Tao, X. Xiao, L. Wang, G. Jiang, F. Zeng, Inhibition of BRD4 suppresses cell proliferation and induces apoptosis in renal cell carcinoma. Cell. Physiol. Biochem. 41, 1947–1956 (2017)
J. Zuber, J. Shi, E. Wang, A.R. Rappaport, H. Herrmann, E.A. Sison, D. Magoon, J. Qi, K. Blatt, M. Wunderlich, M.J. Taylor, C. Johns, A. Chicas, J.C. Mulloy, S.C. Kogan, P. Brown, P. Valent, J.E. Bradner, S.W. Lowe, C.R. Vakoc, RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 478, 524–528 (2011)
A. Chaidos, V. Caputo, K. Gouvedenou, B. Liu, I. Marigo, M.S. Chaudhry, A. Rotolo, D.F. Tough, N.N. Smithers, A.K. Bassil, T.D. Chapman, N.R. Harker, O. Barbash, P. Tummino, N. Al-Mahdi, A.C. Haynes, L. Cutler, B. Le, A. Rahemtulla, I. Roberts, M. Kleijnen, J.J. Witherington, N.J. Parr, R.K. Prinjha, A. Karadimitris, Potent antimyeloma activity of the novel bromodomain inhibitors I-BET151 and I-BET762. Blood 123, 697–705 (2014)
J.E. Delmore, G.C. Issa, M.E. Lemieux, P.B. Rahl, J. Shi, H.M. Jacobs, E. Kastritis, T. Gilpatrick, R.M. Paranal, J. Qi, M. Chesi, A.C. Schinzel, M.R. McKeown, T.P. Heffernan, C.R. Vakoc, P.L. Bergsagel, I.M. Ghobrial, P.G. Richardson, R.A. Young, W.C. Hahn, K.C. Anderson, A.L. Kung, J.E. Bradner, C.S. Mitsiades, BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011)
S.B. Baylin, P.A. Jones, Epigenetic determinants of Cancer. Cold Spring Harb Perspect Biol. 8(9) (2016). https://doi.org/10.1101/cshperspect.a019505
Y. Yokoyama, H. Zhu, J.H. Lee, A.V. Kossenkov, S.Y. Wu, J.M. Wickramasinghe, X. Yin, K.C. Palozola, A. Gardini, L.C. Showe, K.S. Zaret, Q. Liu, D. Speicher, J.R. Conejo-Garcia, J.E. Bradner, Z. Zhang, A.K. Sood, T. Ordog, B.G. Bitler, R. Zhang, BET inhibitors suppress ALDH activity by targeting ALDH1A1 super-enhancer in ovarian Cancer. Cancer Res. 76, 6320–6330 (2016)
N. Tasdemir, A. Banito, J.S. Roe, D. Alonso-Curbelo, M. Camiolo, D.F. Tschaharganeh, C.H. Huang, O. Aksoy, J.E. Bolden, C.C. Chen, M. Fennell, V. Thapar, A. Chicas, C.R. Vakoc, S.W. Lowe, BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 6, 612–629 (2016)
M. Collado, M.A. Blasco, M. Serrano, Cellular senescence in cancer and aging. Cell 130, 223–233 (2007)
R.M. Castilho, C.H. Squarize, L.A. Chodosh, B.O. Williams, J.S. Gutkind, mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 5, 279–289 (2009)
H. Liu, M.M. Fergusson, R.M. Castilho, J. Liu, L. Cao, J. Chen, D. Malide, I.I. Rovira, D. Schimel, C.J. Kuo, J.S. Gutkind, P.M. Hwang, T. Finkel, Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317, 803–806 (2007)
J. Campisi, F. d’Adda di Fagagna, Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007)
D. Hanahan, R.A. Weinberg, The hallmarks of cancer. Cell 100, 57–70 (2000)
Acknowledgments
This work was conducted during a visiting scholar period at the University of Michigan, sponsored by the Capes Foundation within the Ministry of Education, Brazil (grant n. BEX / 88881.132606/2016-01 PDSE). This grant was funded by a University of Michigan School of Dentistry faculty grant, and a Cancer Center Support Grant (P30 CA046592). The authors declare no potential conflicts of interest with respect to authorships and/or publication of this article.
Author information
Authors and Affiliations
Contributions
RLM performed most of the cell culture-based assays, IHC, IF, and participated in the organization of the figures. CHVNF performed the flow cytometry assays, LAR helped with the IHC and IF assays, and LPW helped with the immunofluorescence assays and its quantifications. VZ and MDM contributed to the collection of MEC tissue samples and their clinical data and helped with their re-evaluation. PAV, MAL, CHS and RMC contributed to the conception, design, data organization and writing of the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdiction-al claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Markman, R.L., Webber, L.P., Nascimento Filho, C.H.V. et al. Interfering with bromodomain epigenome readers as therapeutic option in mucoepidermoid carcinoma. Cell Oncol. 42, 143–155 (2019). https://doi.org/10.1007/s13402-018-0416-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-018-0416-2