Abstract
This study aimed to determine the usability of the identified fungus isolated from the marine-mucilage formation in the biotechnological processes. For this purpose, the antimicrobial and antioxidant activities of the extract obtained from the fungus were examined, and the fatty acid methyl ester composition was determined. The molecular identification of the pure fungal culture was made using LSU regions. In addition, fungal extracts were prepared using different solvents, and the antimicrobial activity of these extracts was investigated by disk diffusion and minimum inhibition concentration methods. At the same time, the antioxidant properties of these extracts were analyzed using the DPPH and ABTS free radical removal methods. Also, FAME analysis was performed to determine the fatty acid content of the fungal extract. According to the study results, the new isolate was identified as the fungus Rhizopus stolonifer. Although fungal extracts have no significant antimicrobial activity, it has been determined that they performed successful DPPH and ABTS scavenging activity without needing additional reactions. FAME results indicate that the mucilage-originated fungus R. stolonifer is a valuable provider of fatty acids that, when purified at large bioreactors, can be good and cheap sources of next-generation biologicals for wide-ranging biotechnological applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Marmara Sea is placed between the European and Asian Continents as a basin composed of the Black Sea region’s saline waters and the Mediterranean region’s sub-halocline waters. These two distinct water masses occupy the basin throughout the year. The brackish water forms a relatively thin surface layer (10–15 m thick) with a mean residence time of about 4–5 months, and the sub-halocline waters are separated from the saline ones by a sharp interface (pycnocline) that is about 10–20 m thick [1]. The Marmara Sea, which serves as the transition zone between the Aegean and the Black Sea, is important due to its location. The mucilage formation problem in the Marmara Sea started in April 2021, and there is concern that the mucilage will occur again or spread to other seas [2]. It is assumed that the source of mucilage formation may be the increase in marine organisms consuming organic pollutants. Mucilage, called sea snow, aggregated biomass, foam buildup, flocculation, and mucus creation, is characterized as organic substances primarily generated by marine organisms [3]. The toxins generated by algae, fungi, and bacteria are accountable for the demise or ailment of humans, marine mammals, and other sea creatures. These toxins also give rise to various health and environmental issues, from water discoloration, foam, and deceased marine life to beach pollution. Also, transporting these toxins through the food chain negatively affects marine ecosystems by damaging many aquatic organisms. According to the literature, mucilage formation in marine areas was also observed in countries such as Italy [4]. In addition to biological and ecological problems, the appearance of mucilage negatively affects a wide range of activities, from the fishing industry to the tourism sector, harming the economy [5]. For these reasons, determining and identifying the microorganisms found in the mucilage formation is very important to developing mucilage-fighting strategies. Furthermore, assessing the potential of microbial biomass from microorganisms derived from mucilage offers advantageous prospects for their utilization in biotechnological applications.
In biotechnological uses, certain metabolites synthesized by microorganisms can serve as agents with antimicrobial or antioxidant properties. Microorganisms possess physiological adaptive traits, encompassing bioactive elements they generate to safeguard against other organisms and varying surroundings, consequently exerting a suppressive impact on the proliferation of bacteria, viruses, and fungi. Furthermore, reports indicate fungi’s capacity to produce compounds exhibiting antioxidant capabilities [6]. For instance, fungi have different usage areas due to producing valuable metabolites such as proteins, carbohydrates, fatty acids, vitamins, and minerals that accumulate in their cells [7]. Beyond the range of biotechnologically significant outputs yielded by fungi, fungal-derived products hold considerable benefits due to their affordability and biological compatibility. As an illustration, the initial commercialization of antibiotics in the biotechnological realm included penicillin, a product of fungal origin.
Today, fungi serve as valuable sources for fabricating numerous health-related commodities through biochemical pathways. Fungal products, especially used in the medical sector, have a large market share worldwide due to their high economic value. For example, butyric acid, a fatty acid that fungi can produce [8], can be used as a therapeutic agent in treating diseases such as diabetes and obesity [9]. Additionally, fatty acids obtained from fungi find utility across various commercial sectors, including food, healthcare, and the manufacturing of diverse chemicals like pesticides [10]. Furthermore, the nutrients assimilated by fungi play a pivotal role in shaping intracellular and extracellular metabolites, which hold crucial significance as biotechnological outputs. For instance, the nutrients that fungi ingest are stored as fatty acids. As a result, the fatty acid makeup of fungal species that thrive in nutritionally diverse environments, including those containing mucilage, displays variations attributed to their adaptation to prevailing environmental circumstances [11]. Hence, it holds significance to analyze the fatty acid composition of species isolated from such environments, as this aids in acquiring valuable products. This study aimed to determine the fungal fatty acid content and examine the antimicrobial and antioxidant activities of the fungus newly isolated from the mucilage sea sample and identified by molecular methods. To the best of our knowledge, this is the first study to show the content of the fungus, identified as Rhizopus stolonifer, newly isolated from a mucilage-contaminated marine environment, in terms of various fatty acids of high economic importance. The fungus, newly isolated and identified within the scope of this study, holds significant promise for utilization across diverse commercial sectors due to its abundant reservoir of valuable fatty acids.
2 Materials and methods
2.1 Isolation studies
The seawater samples with mucilage, collected from the Bostancı Coast of Istanbul (Turkey) in June 2021, were taken with plastic water bottles sterilized by dilute sodium hypochlorite solution. Samples were transferred to the laboratory within the same day and spread on Petri plates [12].
The sample was spread on Petri plates containing PDA (potato dextrose agar). The Petri plates were incubated at 30 ± 2 °C for 7 days. The fungal colonies on the plates were isolated and purified by repeatedly streaking the cells on the PDA medium agar plate. The pure cultures were kept at 4 °C and were transferred to PDA media every 3 months [12].
2.2 Molecular identification studies
The isolated fungal cells from an exponentially growing isolate culture were used for molecular identification. The fungal DNA isolation studies were carried out with the EurX GeneMATRIX Plant & Fungi DNA isolation kit (Poland). Spectrophotometric measurement was performed in a Thermo Scientific Nanodrop 2000 (USA) device to control the amount and purity of DNA obtained after DNA isolation.
The PCR study amplified gene regions targeted for species identification with the primers LROR (forward), 5′-(ACCCGCTGAACTTAAGC-3′ and LR5 (reverse): 5′-TCCTGAGGGAAACTTCG-3′ in LSU locus [13]. Also, the PCR conditions are given in Table 1.
The amplification results obtained by PCR (kyratec thermocycler) were carried out in 1.5% agarose gel prepared with 1 × TAE buffer at 100 Volt current for 90 min, and their images were taken in UV light using ethidium bromide dye (Fig. 1). One-step PCR was performed to amplify the region of approximately 600 bases. The PCR reaction was performed using Solis Biodyne (Estonia) FIREPol® DNA Polymerase Taq polymerase enzyme. After PCR, a single band was obtained on the agarose gel, and the success of the PCR process was checked. During the purification step of the PCR product, the obtained single band samples were purified according to the kit’s procedures using the MAGBIO “HighPrep™ PCR Clean-up System” (AC-60005) purification kit. For Sanger sequencing, the ABI 3730XL Sanger sequencing device (Applied Biosystems, Foster City, CA, USA) and the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) were used in the Macrogen Netherlands laboratory. CAP contig assembly algorithm was used in BioEdit software to perform this process.
2.3 Extract preparation
The isolated and identified fungus was grown in potato dextrose broth (PDB) media, and the biomass was harvested by filtration, washed twice with distilled water, and then dried in an oven at 60 °C [14]. After drying, 4 g of powdered biomass was weighed and poured into cartridges of blotting paper closed with a stapler. The cartridges were hung inside the 250-mL flasks containing 100 mL of ethanol and kept in an incubator at 35 °C with a 150-rpm shaker for 24 h. The cartridges were taken into Petri dishes to evaporate ethanol. The same processes were repeated with methanol, the most commonly used solvent. After the processes with each solvent, 0.16 g of extract was obtained, so the extraction efficiency percentage was calculated as 4%. Stock fungus extracts with a concentration of 1 mg/mL (1000 ppm) were prepared and stored at + 4 °C.
2.4 Antimicrobial activity studies
2.4.1 Disk diffusion method
Firstly, the disk diffusion method [15] was used to test the antimicrobial activity of the obtained extract. The fungal extract was impregnated with 6-mm-diameter sterile blank discs and dried in an oven at 30 °C for 30 min. The test organisms having different cell structures listed in Table 2 were used for antimicrobial activity testing. Muller Hinton Agar-Broth (MHA-MHB) and Sabouraud Dextrose Agar-Broth (SDA-SDB) were used for bacteria and yeasts, respectively. The bacterial and yeast strains were adjusted to Mc Farland 0.5 (1.5 × 108) and Mc Farland 2 (6 × 108), respectively. The discs impregnated with sterile distilled water were used as a negative control, and the discs impregnated with commercial antibiotics azithromycin (for bacteria) and voriconazole (for yeasts) were used as a positive control. Then, bacteria and yeasts were incubated at 37 °C for 24 h and at 30 °C for 48 h in an oven, respectively. At the end of the incubation period, it was observed whether inhibition zones were formed around the discs, and the inhibition zones formed around the discs were measured using a caliper. Trials were carried out under aseptic conditions and in two parallels, and the tests were repeated twice to determine their accuracy [16].
2.4.2 Minimum inhibition concentration
Minimum inhibitory concentration (MIC) tests will be performed using 96-well microplates and microdilution standard methods for bacteria and yeasts [17, 18], respectively. A series of test tubes were prepared from the extracts at different concentrations. Wells of sterile 96-well microplates were prepared by adding MHB (bacteria)/SDB (yeast), an inoculum of tested microorganism given in Table 2 (1.5 × 108 CFU/mL bacteria/6 × 108 CFU/mL yeast), and fungal extract. Stock fungal extract solutions were used, and the fungal extract concentration was adjusted to 1250 µg/mL in the first well; then, serial dilutions were made in a 1: 2 ratio into the subsequent wells. Plates were covered with sterile lids and incubated for 24 h at 37 ± 0.1 °C for bacteria and 48 h at 30 ± 0.1 °C for yeasts. All extracts have been tested twice against each organism. The MIC value was determined as the lowest concentration of the extracts that killed the microorganisms, and standard antibiotics (azithromycin for bacteria and voriconazole for yeasts) were used as positive controls.
2.5 Antioxidant activity assays
Several methods have been developed for the determination of antioxidant activity. Important in vitro methods for assessing antioxidant capacity include the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS∙ +) radical scavenging assay and the 1,1-diphenyl-2-picrylhydrazyl (DPPH·) radical scavenging assay [19].
2.5.1 Preparation of solutions used in ABTS∙ + scavenging activity
Phosphate buffer (0.1 M) at pH 7.4
Dissolve 2.84 g Na2HPO4 in 150 mL of distilled water. Adjust the pH to 6.6 using a pH meter. Finally, bring the volume to 200 mL with distilled water.
ABTS (2 mM) solution
Stir 11 mg of ABTS in 0.1 M phosphate buffer at pH 7.4 overnight until completely dissolved. Bring the volume to 100 mL with distilled water.
Potassium persulfate (2.5 mM) solution
Stir 66.25 mg K2O8S2 with a magnetic stirrer in 0.1 M phosphate buffer at pH 7.4 until completely dissolved. Bring the volume to 100 mL with distilled water.
2.5.2 Preparation of solution used in DPPH· free radical scavenging activity
DPPH (10–3 M) solution
Forty milligrams of DPPH was dissolved entirely in 100 mL of 96% ethanol by stirring with a magnetic stirrer overnight in a beaker covered with aluminum foil.
2.5.3 Determination of ABTS∙ + scavenging activity
ABTS radical scavenging activity was determined using a commonly used method [20]. First, a 7-mM ABTS solution was prepared, and then, ABTS radicals were generated by adding a 2.5-mM persulfate solution. As a control, 500 µL of ABTS radical solution and 1500 µL of 96% ethanol were used. Before using the ABTS radical solution, the absorbance of the control solution was adjusted to 0.900 ± 0.025 nm at 734 nm using a 0.1 M phosphate buffer at pH 7.4. For the evaluation of ABTS radical scavenging activity, different concentrations (10–20-40 µg/mL) of samples (E1, E2, E3, E4) were added to 0.5 mL of ABTS radical solution, and the final volume was adjusted to 2.5 mL with 96% ethanol. After incubating in the dark for 30 min, the absorbance values at 734 nm against a blank of ethanol were measured and recorded. A decrease in absorption was observed as the sample concentrations increased. The experiments were conducted with two biological replicates, each with three technical replicates.
2.5.4 Determination of DPPH· free radical scavenging activity
The DPPH free radical scavenging assay was performed using the Blois method [21]. A 1-mM DPPH solution was prepared immediately before the experiment and used. E1, E2, E3, and E4 samples were pipetted into test tubes from their stock solutions, with concentrations of 10, 20, and 40 µg/µL, respectively, and the final volume was adjusted to 2 mL with 96% ethanol. Then, 0.5 mL of freshly prepared DPPH solution was added to the tubes containing the samples and standard substances, and the tubes were vortexed. After incubating the tubes in the dark for 30 min, the absorbance values at 517 nm against a blank of ethanol were measured, and the results were recorded. Two milliliters of ethanol and 0.5 mL of DPPH solution were used as a control. The decreasing absorbance value indicates the amount of DPPH radical scavenged. The experiments were conducted with two biological replicates, each with three technical replicates.
2.6 Fatty acid methyl ester (FAME) analysis
2.6.1 Sample preparation
H2SO4 and heneicosanoic acid precipitated in heptane were added to 10 mg of Rhizopus stolonifer. It was treated with a thermomixer at 750 rpm at 80 °C for 2 h. After 2 h, NaCl solution and hexane were added and mixed with vortex for 20 s. Refrigerated centrifugation was done at 3000 g (5000 rpm) for 3 min at 20 °C. With phase separation, the supernatant was collected in a vial.
2.6.2 Chromatography protocol
The experiments were conducted using GC-2010 PLUS equipped with FID (Shimadzu, Japan). One microliter of the sample was injected at 250 °C using split mode (ratio 1/10) through the autosampler into the system. The TRB-WAX capillary column 30 m × 0.53 mm × 1.00 µm (TR-131035/Teknokroma, Spain) was installed for FAME analysis. The flow control mode was pressure, and the run was completed in 47 min. Supelco 37 Component FAME Mix (Supelco, USA) was used as a quantitative standard. The standard calibration curve for each of these reference fatty acids was plotted. Subsequently, the FAME concentrations in the samples were calculated from the calibration curves of these reference fatty acids [22].
3 Results and discussion
3.1 Identification of fungus
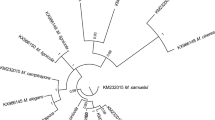
The fungus isolated from mucilage in seawater of the Marmara Sea is identified as Rhizopus stolonifer using both morphological and molecular techniques. The morphology of the newly isolated is given in Fig. 2. The fungus has a mycelial structure and a cotton-candy-like texture (Fig. 2a). Initially, the colony is white and then turns gray to yellowish brown (Fig. 2a and b). There are black round sporangia at the upper end of non-septate white filaments (Fig. 2c). Rhizopus stolonifer, commonly known as black bread mold, is a member of Zygomycota and has a widespread ecology [23]. It is an important species that feeds on saprophytes. R. stolonifer can be used in various biotechnological processes such as enzyme production [24].
Radical scavenging activities for different extracts of Rhizopus stolonifer. a ABTS and b DPPH free radical scavenging activity plots. c The comparison of ABTS and DPPH scavenging capacities (%) for different extracts of Rhizopus stolonifer in 40 µg/mL concentration. Control, ABTS or DPPH where applicable; E1, Rhizopus stolonifer extract in diethyl ether; E2, Rhizopus stolonifer extract in dichloromethane; E3, Rhizopus stolonifer extract in methanol; E4, Rhizopus stolonifer extract in ethanol
3.2 Antimicrobial activity
The antimicrobial activity of R. stolonifer extracts was tested on five different structured microorganisms using the disk diffusion method. Bacillus subtilis and Staphylococcus aureus represent gram-positive bacteria; Escherichia coli represent gram-negative bacteria; Candida albicans and Candida krusei represent the yeasts (Table 2). As a result of the agar disc diffusion assays, the inhibition zone diameters with discs having azithromycin were 26.75 ± 3.59 and 27.75 ± 2.87 against E. coli and S. aureus, respectively. The inhibition zone diameters with voriconazole discs were determined as 35 ± 5.00 and 34 ± 1.41 for C. albicans and C. krusei, respectively. However, no inhibition zone was observed with discs having fungal extracts (all extracts) for all test organisms. MIC (minimum inhibitory concentration) tests of higher sensitivity were also conducted on all fungal extract samples [25]. The MIC values are given in Table 3. As can be seen in the table, the extracts do not have a significant antimicrobial effect. However, ethanol and methanol extracts seem to slightly affect S. aureus, a gram-positive test bacterium, compared with other bacteria. Its impact on gram-positive, which does not have a lipopolysaccharide layer, is related to the interaction of the substances in the extract [26]. The antimicrobial effect may be due to the fatty acids or other compounds contained in the fungus extract [27]. No research has been found in the literature showing the antimicrobial effect of extracts of R. stolonifer prepared with the solvents used in this study. It is known that solvent polarity used in extraction affects the presence of different substances in the extract [28]. For this reason, solvents with different polarities, such as ethanol, diethyl ether, and dichloromethane, have been tested in this study. However, no significant antimicrobial effect was detected.
3.3 Antioxidant activity
Antioxidants provide electrons or hydrogen to free radicals, thereby reducing them and preventing their negative impact on health in a certain way [29]. These molecules, mainly present in the human body, serve a protective function by donating their electrons to eliminate free radicals without becoming free radicals themselves [19,20,21,22,23,24,25,26,27,28,29,30]. The presence of compounds or systems that exhibit antioxidant effects in living organisms is a crucial requirement for life. These antioxidant molecules are responsible for various biological functions such as anticarcinogenic, antimutagenic, and antiaging effects [31]. The mechanisms of action of antioxidant substances occur by influencing reactive oxygen species (ROS), either by capturing them or converting them into weaker and different compounds. Antioxidants interact with ROS, facilitating the transfer of an electron or hydrogen, thereby reducing their activity or causing them to become inactive. Molecules with antioxidant activity prevent damage caused by free radicals by disrupting chain reactions through binding to ROS, thus inhibiting their functions [32]. Recent studies have demonstrated that antioxidants protect biological systems against oxidative stress.
The ABTS∙ + radical scavenging activity of samples obtained from different solutions, namely E1 (Rhizopus stolonifer extract in diethyl ether), E2 (Rhizopus stolonifer extract in dichloromethane), E3 (Rhizopus stolonifer extract in methanol), and E4 (Rhizopus stolonifer extract in ethanol), was compared. When compared to the standard antioxidants Trolox and BHT (butylated hydroxytoluene), it was observed that E1, E2, E3, and E4 samples showed similar values in scavenging the ABTS∙ + radical. Specifically, the radical scavenging activity of E1 and E3 samples was close to that of the standard antioxidants. It is determined that R. stolonifer extracts exhibited antioxidant properties to a certain extent (Fig. 2a).
The DPPH radical scavenging activity of E1, E2, E3, and E4 samples at different concentrations was also monitored. The E2 and E4 samples showed more pronounced activity than the others based on the decreasing absorbance values. The radical scavenging rate increased with increasing concentrations of the samples (Fig. 2b). Furthermore, the ABTS∙ + and DPPH radical scavenging percentages of E1, E2, E3, and E4 samples at a concentration of 40 µg/mL were compared. Based on the determined percentage values, E3 and E4 samples exhibited a balanced percentage of radical scavenging (Fig. 2c).
IC50 values of extracts are shown in Table 4. As known, lower IC50 values indicate stronger antioxidant activity, meaning the substance is more effective at lower concentrations. Accordingly, E1 has moderate antioxidant activity in both assays (DPPH IC50, 43.31 µg/mL; ABTS IC50, 28.87 µg/mL), being more effective in the ABTS assay than in the DPPH assay. E2 shows relatively better antioxidant activity in the DPPH assay (IC50, 25.66 µg/mL) compared to the ABTS assay (IC50, 36.47 µg/mL), but it is less effective than E1 in the ABTS assay. E3 has moderate antioxidant activity, performing slightly better in the ABTS assay (IC50, 27.72 µg/mL) compared to the DPPH assay (IC50, 31.50 µg/mL). E4 demonstrates better antioxidant activity in the DPPH assay (IC50, 27.72 µg/mL) compared to the ABTS assay (IC50, 32.99 µg/mL), similar to E2 but slightly less effective overall.
In a research endeavor, the green algae Botryococcus braunii was harnessed to produce palladium and platinum nanoparticles. The investigation centered around the capacity of the fungal extract to counteract DPPH free radicals. The fungus exhibited its activity after incorporating palladium and platinum elements [33]. Furthermore, fungi were employed in the biogenic synthesis of platinum nanoparticles, and the result was examined for its free radical scavenging capacity [34]. Following the synthesis of three novel butenolide compounds from fungi, original chemical entities were derived. Most of these compounds demonstrated strong antioxidant properties by scavenging DPPH and ABTS free radicals, highlighting the potent antioxidant nature of butenolides [35]. A previous study confirmed the ABTS and DPPH free radical scavenging abilities of indigenous endophytic fungi, in which the molecular identification, profiling of volatile metabolites, and assessment of bioactivities were also studied [36].
In a study conducted to increase the bioactivity potential, edible Rhizopus strains were used, and fermentation was performed. In the study, the use of Rhizopus oligosporus (ATCC 64063, DSM 1967, NRRL 2710) and R. oryzae (CBS 372.63) strains increased the concentration of bioactive components in pumpkin seed cake. R. oligosporus ATCC 64063 was considered the most effective strain among the tested fungal strains. Pumpkin seed cake fermented with this strain for 2 days was characterized by a 368% higher ABTS˙ + scavenging activity [37]. Another study used DPPH to measure the antioxidant activity of different types of tempeh, a traditional Indonesian dish produced from soybeans fermented by R. microsporus. Tempeh was produced using various microorganisms under laboratory conditions. The antioxidant activities of tempeh from different producers were found to vary. As a result, it was observed that the fermentation process of soybeans increased antioxidant activity. Depending on this diversity, it was observed that some groups of tempeh had the highest antioxidant activity. In conclusion, the tempeh fermentation process increases antioxidant activity to varying degrees (approximately 52–76%), which can vary depending on the types of microorganisms used and the fermentation duration [38]. A comparative study was conducted on the ABTS + radical scavenging activity of Acremonium charticola isolated from fermented and dried mushrooms commonly used in Indonesia, R. oryzae fungus, and ascorbic acid. It was observed that A. charticola, R. oryzae, and ascorbic acid exhibit ABTS + radical scavenging activities of 93.11%, 14.20%, and 77.63%, respectively. As seen, A. charticola showed the highest removal rate. This research has demonstrated that fungi can also possess antioxidant activity and may serve as a source of antioxidant compounds [39].
In a study that utilized the green alga Botryococcus braunii for the synthesis of palladium and platinum nanoparticles, the DPPH radical scavenging activity of the mushroom extract was examined. The mushroom’s activity was observed after the use of palladium and platinum elements [33]. Additionally, fungi were employed in the biogenic synthesis of platinum nanoparticles, and the synthesized products exhibited free radical scavenging activity [34]. After isolating three new butenolide compounds synthesized from mushrooms, new chemical compounds were obtained. It was demonstrated that most of these compounds are potent antioxidant agents, with an average of 30% scavenging activity for DPPH and an average of 10% scavenging activity for ABTS [35]. Additionally, a recent study was conducted on the molecular identification, volatile metabolite profiling, and bioactivities of an endophytic fungus. In this study, significant scavenging activity was observed at a concentration of 100 µg/mL, with rates of 61.53% for DPPH free radicals and 85.62% for ABTS free radicals [36].
Within our study, it was found that the highest ABTS free radical scavenging activity was 35.12 ± 0.35%, and the highest DPPH free radical scavenging activity was 36.23 ± 0.31%. During the extraction process, four different solvents diethyl ether (E1), dichloromethane (E2), methanol (E3), and ethanol (E4) were used. Among these solvents, the E3 solution showed the highest ABTS free radical scavenging activity at 35.12 ± 0.35%, while the E2 solution was the most suitable for DPPH free radical scavenging activity at 36.23 ± 0.31%. In terms of IC50 values; E2 with an IC50 value of 25.66 µg/mL is the most effective extract in the DPPH assay, indicating it has the strongest antioxidant activity among the extracts tested. However, E3 with an IC50 value of 27.72 µg/mL is the most effective extract in the ABTS assay, showing it has the strongest antioxidant activity in this assay. Considering both assays, E3 emerges as the most effective extract overall. Although E2 has the lowest IC50 in the DPPH assay, E3 has a lower IC50 in the ABTS assay and a comparable IC50 in the DPPH assay, making it the most consistently effective extract across both types of antioxidant activity assays.
Overall, the extraction of the fungus yielded discernible activities in scavenging DPPH and ABTS free radicals, all without necessitating supplementary reactions. The stability of our study has been ensured, providing a guiding framework for future research. Thus, an advantageous process has been established for the determination of antioxidant activity.
3.4 Fatty acid methyl ester composition
Fungi obtain nutrients by breaking down and converting organic matter, whether alive or dead, into substrates they can use. This includes fatty acids, which fungi can store inside their cells. Fungi can change their shape, physiology, and behavior in response to various environmental factors such as temperature, moisture levels, nutrition availability, and proximity to other fungi. Fungi are also known for their ability to produce and store fatty acids, which can benefit certain types of fungi grown for their oil [27]. The fatty acid composition of R. stolonifer extract is shown in Table 5. A total of 28 fatty acids were identified in the content of the extract; however, eight were found in high concentrations. As shown in Fig. 3, the most abundant fatty acid was butyric acid, with approximately 557 mg/mL. Moreover, lauric (369 µg/mL) and caproic (314 µg/mL) acids were also found in very high concentrations (Fig. 4a, b). R. stolonifer extract also contains high concentrations of myristic (286 µg/mL), pentadecanoic (205 µg/mL), caprylic (164 µg/mL), tridecanoic (159 µg/mL), and palmitic (51 µg/mL) acids (Fig. 4c, d and Fig. 5).
These results indicate that the mucilage-isolated fungus R. stolonifer is a valuable provider of fatty acids that, when purified at large bioreactors, can be good and cheap sources of next-generation biologicals for wide-ranging biotechnological applications.
Fungal fatty acids refer to the fatty acids produced and stored by fungi. Fungi can synthesize various fatty acids, including saturated, monounsaturated, and polyunsaturated. These fatty acids can be stored inside the fungal cells and used as a source of energy and nutrition when other nutrients are scarce. Some fungi have been found to produce high levels of specific fatty acids, making them potential sources for producing biofuels and other industrial products. Fungal fatty acids may have various biological and medical applications, such as antimicrobial and antitumor properties [40, 41]. Additionally, it has been shown in previous studies that microbial metabolism is directed to butyric acid production in environments containing carbohydrate-rich wastes [42]. In this study, we found that the fungus has a tendency to produce high amounts of butyric acid in an environment rich in organic matter, such as mucilage. The production of butanol, a green alternative solution to fossil fuels, uses butyric acid (C:4) as a substrate [43]. Butyric acid, a short-chain fatty acid, is emerging as one of the renewable green C4 platform chemicals that can give an alternative solution to fossil fuels and reduce health and environment-related issues.
Studies suggest that linoleic acid and α-linoleic acid are vital for consumption because the human body cannot produce them independently. Although these compounds can be found in many different organisms, fungi are a valuable source of fatty acids that offer additional advantages. [44]. Fungi have been reported to be rich and global sources of fatty acids such as myristic, palmitic, palmitoleic, stearic, oleic, linoleic, α-linoleic, arachidic, and eicosenoic acids [45]. Research conducted on different fungi from Turkey found that the most prevalent fatty acids were linoleic, palmitic, oleic, stearic, and arachidic acids [46]. Another study from Turkey identified caproic, caprylic, lauric, myristic, pentadecanoic, palmitic, tridecanoic, etc. fatty acids among 37 fatty acids in the composition of Caesar’s fungi [47].
Ensuring a balanced intake of essential fatty acids, with a 1:1 or 2:1 between omega-6 and omega-3, may aid in preventing obesity since an imbalanced ratio of these fatty acids has been linked to adipogenesis. Moreover, essential fatty acids are involved in the formation of high-density lipoprotein (HDL), which facilitates the transportation of fat from the bloodstream to the liver, where it can be metabolized, thus reducing the risk of cardiovascular diseases. Additionally, EPA and DHA, two omega-3 fatty acids, can alter the structure of cell membranes, protein functions, the production of lipid mediators, and gene expression patterns, thereby improving overall health [45]. Butyrate is famous as a potential therapeutic agent for various conditions, including inflammatory bowel disease, type 2 diabetes, and obesity. It is a short-chain fatty acid (SCFA) that contains four carbon atoms and is produced in the colon.
Along with other SCFAs, such as acetate and propionate, butyrate is a vital energy source for the cells lining the colon. It also plays a crucial role in regulating the immune system and reducing inflammation in the gut [48]. Butyrate has also shown a strong negative and dose-dependent effect on the biofilm formation of fungi [49]. On the other hand, medium-chain fatty acids (MCFAs) are types of fatty acids that can be either saturated or unsaturated and contain between 6 and 12 carbon atoms. Some examples of MCFAs include caproic acid (C6:0), caprylic acid (C8:0), capric acid (C10:0), and lauric acid (C12:0) [50]. The fatty acid composition of fungi differs among species and is affected by different intrinsic and external factors [44]. The previous study investigated the composition of fatty acids in various species of macrofungi. The presence of numerous fatty acids such as caproic, caprylic, capric, undecanoic, lauric, tridecanoic, myristoleic, myristic, pentadecanoic, palmitoleic, palmitic, cis-10-heptadecenoic, heptadecanoic, γ-linolenic, linoleic, oleic, stearic, arachidonic, eicosapentaenoic, eicosatrienoic, eicosadienoic, eicosaenoic acid, α-linolenic, arachidic, heneicosanoic, erucic, behenic, tricosanoic, nervonic, and lignoceric acids was detected in the range from approximately 30 to 3175 mg/kg [51]. Researchers have previously reported on the antimicrobial properties of several types of fatty acids, including tridecanoic acid, tetradecanoic acid, and pentadecanoic acid. Tridecanoic acid, in particular, has been found to possess several biological activities, such as anthelminthic, anti-inflammatory, antimicrobial effects, and anticancer properties. It has been proposed that tridecanoic acid could be used as an agent to control plant and human diseases [52]. Pentadecanoic acid is a type of fatty acid not naturally produced by the body but can be consumed as part of the diet. Studies have shown that this fatty acid has various health benefits, including anti-inflammatory and antifibrotic effects. It also stabilizes red blood cells and helps repair damaged mitochondria. By lowering cholesterol, triglycerides, and glucose levels, pentadecanoic acid may reduce inflammation, anemia, and liver fibrosis, leading to improved health outcomes for conditions associated with the heart, metabolism, liver, and aging [53].
Caproic acid, a versatile chemical compound, can now be generated at high rates and specificities from low-grade mixed organic waste through lab- and pilot-scale systems [54]. Caproic acid has various applications, such as being used directly as feed additives, antimicrobials, and plant growth promoters. It can also serve as a precursor to producing commodities, including lubricants, fragrances, paint additives, and pharmaceuticals. Commercially available caproic acid produced from food crops is costly due to these crop oils’ low caproic acid content. Therefore, processes have been developed using mixed organic waste as a feedstock via microbial fermentation, specifically through chain elongation via a reversed β-oxidation pathway. This approach utilizes short-chain fatty acids like acetate and butyrate, intermediates from the anaerobic degradation of mixed organic waste, to yield caproate as the dominant end-product with high production rates and specificity. This process provides a more economical and sustainable way of producing caproic acid for industrial use [54].
Caprylic acid is also an all-purpose compound with remarkable properties, making it applicable in various fields, including health promotion, disease control, cosmetics, and industry. It is commonly consumed as a dietary supplement and has been suggested to aid in weight management by increasing calorie burning in the body, as indicated in some studies. Caprylic acid is also part of a ketogenic diet to manage intractable epilepsy in children [55]. Caproic, caprylic, and capric acids exhibited potent anticancer properties by decreasing the viability of colon cancer cells by 70 to 90%. These acids were also observed to down-regulate genes that regulate the cell cycle and up-regulate genes involved in apoptosis [56]. Similar results were seen in a human skin epidermoid carcinoma cell line (A-431) [57]. Another in vitro study demonstrated that lauric acid, when administered at a dosage of 0.5 mM, induced apoptotic changes and cell cycle arrest in the G0/G1 and G2/M phases. This treatment also increased intracellular reactive oxygen species levels while decreasing intracellular glutathione levels [58]. Lauric acid also significantly inhibited human hepatocellular (HepG2) proliferation and murine macrophage (Raw 264.7) cells. However, the extent of the impact varied depending on the nature and origin of the cells [59]. Moreover, lauric acid stimulated apoptosis in endometrial cancer cells (Ishikawa) by activating EGFR phosphorylation events [56,57,58,59,60].
Several types of fatty acids, including capric, lauric, palmitic, oleic, linoleic/linolenic, and eicosadienoic acid, have demonstrated antibiofilm properties against microbial biofilms of S. aureus and fungal C. albicans. Additionally, various derivatives of fatty acids play a critical role as cell-to-cell signals in many plant-associated bacteria [61]. The antibacterial effects of palmitic acid were also proven against different pathogens [62]. A recent study found that saw palmetto oil, which contains high levels of lauric acid and myristic acid, can prevent the formation of biofilms in single, dual, and three-species biofilm models of S. aureus, E. coli, and C. albicans. Notably, this inhibition of biofilm formation occurred without any impact on the growth of the individual cells of these microorganisms in a liquid environment [61]. Reduced levels of diacylglycerol kinase in skeletal muscles have been associated with a decrease in glucose uptake and are closely linked to the development of type 2 diabetes. Therefore, increasing the expression of diacylglycerol kinase is believed to be a possible way to improve glucose regulation and protect against diabetes. Recent findings provide strong evidence that myristic acid can enhance glucose uptake in myotubes and improve skeletal muscle mass and that these effects depend on the expression of diacylglycerol kinase [63]. Capric acid, myristic acid, lauric acid, and palmitic acid were combined in binary mixtures and evaluated as possible phase-change biomaterials to improve the thermal performance of concrete [64].
4 Conclusion
In this study, the fungus was isolated from the sample taken from the mucilage formed due to pollution in the Marmara Sea and identified using LSU gene regions. According to the results of molecular identification, it was understood that the new isolate was R. stolonifer. Extracts of the new isolate fungus were prepared using solvents with different properties, and their antimicrobial and antioxidant activities were investigated. The extracts were determined to be ineffective in antimicrobial activity and showed effective antioxidant activity. Also, the fatty acid profile of the new isolate was examined, and it was determined that the fungus can produce fatty acids such as butyric acid, lauric acid, and caproic acid at high rates, which can be used in different biotechnological applications. In conclusion, this study shows that the fungus isolated from the mucilage formed due to marine pollution can be used as a rich source of fatty acids.
Data availability
The raw data files are available on request from the corresponding author.
References
Toklu-Alıçlı B, Balkıs N, Toklu AS, Höbek A (2010) First record of Amphorellop sistetragona (Protozoa: Ciliophora: Tintinnina) from the sea of Marmara. Eur J Biol 69(2):103–106
Karadurmuş U, Sarı M (2022) Marine mucilage in the Sea of Marmara and its effects on the marine ecosystem: mass deaths. Turk J Zool 46(1):93–102. https://doi.org/10.3906/zoo-2108-14
Özalp HB (2021) First massive musilage event observed in deepwaters of Çanakkale strait (Dardanelles), Turkey. J Black Sea/Medit Environ 27(1):49–66
Mecozzi M, Pietroletti M, Scarpiniti M, Acquistucci R, Conti ME (2012) Monitoring of marine mucilage formation in Italian seas investigated by infrared spectroscopy and independent component analysis. Environ Monit Assess 184:6025–6036. https://doi.org/10.1007/s10661-011-2400-4
Karlson B, Andersen P, Arneborg L, Cembella A, Eikrem W, John U, West JJ, Klemm K, Kobos J, Lehtinen S, Lundholm N, Mazur-Marze H, Naustvoll L, Poelman M, Provoost P, Rijcke M, Suikkanen S (2021) Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algea 102. https://doi.org/10.1016/j.hal.2021.101989
Vitale GA, Coppola D, Palma Esposito F, Buonocore C, Ausuri J, Tortorella E, de Pascale D (2020) Antioxidant molecules from marine fungi: methodologies and perspectives. Antioxidants 9(1183):1–36. https://doi.org/10.3390/antiox9121183
Husna Hussain A, Shah M, Hamayun M, Qadir M, Iqbal A (2022) Heavy metal tolerant endophytic fungi Aspergillus welwitschiae improves growth, ceasing metal uptake and strengthening antioxidant system in Glycinemax L. Environ Sci Pollut Res 29:15501–15515. https://doi.org/10.1007/s11356-021-16640-1
Jiang L, Fu H, Yang HK, Xu W, Wang J, Yang ST (2018) Butyric acid: applications and recent advances in its bioproduction. Biotechnol Adv 36(8):2101–2117. https://doi.org/10.1016/j.biotechadv.2018.09.005
Van Deuren T, Blaak EE, Canfora EE (2022) Butyrate to combat obesity and obesity-associated metabolic disorders: current status and future implications for therapeuticuse. Obes Rev 23(10):e13498. https://doi.org/10.1111/obr.13498
Zhan XY, Li B, Huang BC, Wang FB, Zhang YQ, Zhao SG, Li M, Wang HY, Yu XJ, Liu XY, Jiang J, Wang ZP (2022) Production, biosynthesis, and commercial applications of fatty acids from oleaginous fungi. Front Nutr 9:873657. https://doi.org/10.3389/fnut.2022.873657
Suastes-Rivas JK, Hernández-Altamirano R, Mena-Cervantes VY (2020) Efficient production of fatty acid methylesters by a waste water-isolated microalgae-yeast co-culture. Environ Sci Pollut Res 27:28490–28499. https://doi.org/10.1007/s11356-019-07286-1
Taştan BE (2017) Clean up fly ash from coal burning plants by new isolated fungi Fusarium oxysporum and Penicillium glabrum. J Environ Manage 200:46–52. https://doi.org/10.1016/j.jenvman.2017.05.062
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Salinas-Salazar C, Garcia-Perez JS, Chandra R, Castillo-Zacarias C, Iqbal HMN, Parra-Saldívar R (2019) Methods for extraction of valuable products from micro algae biomass. In: Alam MdA, Wang Z (ed) Microalgae biotechnology for development of biofuel and wastewater treatment, 1st edn. Springer, Singapore, pp 245–263. https://doi.org/10.1007/978-981-13-2264-8_11
CLSI (ed) (2015) Clinical and Laboratory Standards Institute: performance standards for antimicrobial disk susceptibilitytests; approved standard, 12th edn. Wayne, PA, USA. https://clsi.org/media/1631/m02a12_sample.pdf. Accessed 13 Mar 2024
Prakash JW, Marimuthu J, Jeeva S (2011) Antimicrobial activity of certain fresh water microalgae from thamirabarani river, Tamil Nadu, South India. Asian Pac J Trop Biomed 1(2):170–173. https://doi.org/10.1016/S2221-1691(11)60149-4
CLSI M07-A9 (2012) Clinical and Laboratory Standards Institute: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 9th edn. Wayne, PA, USA. https://clsi.org/media/1928/m07ed11_sample.pdf. Accessed 13 Mar 2024
NCCLS (2002) Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd edn. NCCLS document M27-A2. NCCLS, Wayne
Çetinkaya Y, Göçer H, Menzek A, Gülçin I (2012) Synthesis and antioxidant properties of (3,4-dihydroxyp(henyl)(2,3,4- trihydroxyphenyl) methanone and its derivatives. Arch Pharm 345(4):323–334. https://doi.org/10.1002/ardp.201100272
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26(9–10):1231–1237. https://doi.org/10.1016/s0891-5849(98)00315-3
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Taştan BE, Tekinay T (2016) A novel coal additive from microalgae produced from thermal power plant flue gas. J Clean Prod 133:1086–1094. https://doi.org/10.1016/j.jclepro.2016.06.053
Gould AB (2009) Fungi: plantpathogenic. In: Schaechter M (ed) Encyclopedia of microbiology, 3rd edn. AcademicPress, San Diego, USA, pp 457–477. https://doi.org/10.1016/B978-012373944-5.00347-3
Galiana CP, Miralles-Robledillo JM, Bernabeu E, Harfi N, Martínez-Espinosa RM (2022) Genetic and protein engineering of halophilicenzymes. In: Arora NK, Agnihotri S, Mishra J (eds) Extremozymes and their industrial applications, 1st edn. Academic Press, India, pp 249–278. https://doi.org/10.1016/B978-0-323-90274-8.00003-4
Gül ÜD, Cantürk Z, İlhan S, Birgi F (2023) The bioactive properties of the bryophyte sample collected from Bilecik (Turkey) Province. S Afr J Bot 156:91–98. https://doi.org/10.1016/j.sajb.2023.03.012
Paracini N, Schneck E, Imberty A, Micciulla S (2022) Lipopolysaccharides at solid and liquid interfaces: models for biophysical studies of the gram-negative bacterial outer membrane. Adv Coll Interface Sci 301:102603. https://doi.org/10.1016/j.cis.2022.102603
Hao G, Barker GC (2022) 9 Fatty acid secretion by the white-rot fungus, Trametesversicolor. Journal of Industrial Microbiology and Biotechnology 49(1). https://doi.org/10.1093/jimb/kuab083
Garcia-Larez FL, Murillo-Hernandez JL, Vargas-Sanchez RD, Torrescano-Urrutia GR, Torres-Martinez BM, Sánchez-Escalante A (2021) Effect of extraction solvent on metabolites content, antioxidant, and antibacterial activity of coffee bagasse. TIP Revista Especializada en Ciencias Químico-Biológicas 24:1–10. https://doi.org/10.22201/fesz.23958723e.2021.363
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 4(8):118–126. https://doi.org/10.4103/0973-7847.70902
Prior RL, Cao G (2000) Antioxidant phytochemicals in fruits and vegetables: diet and health implications. HortScience 35(4):88–592. https://doi.org/10.21273/hortsci.35.4.588
Cook NC, Samman S (1996) Flavonoids -Chemistry, metabolism, cardio protective effects, and dietary sources. J Nutr Biochem 7(2):66–76. https://doi.org/10.1016/0955-2863(95)00168-9
Kalin P, Gülçin İ, Gören AC (2015) Antioxidant activity and polyphenol content of cranberries (Vaccinium macrocarpon). Rec Nat Prod 9(4):496
Anju ARYA, Gupt K, Chundawat TS (2020) In vitro antimicrobial and antioxidant activity of biogenically synthesized palladium and platinum nanoparticles using Botryococcus braunii. Turk J Pharm Sci 17(3):299–306. https://doi.org/10.4274/tjps.galenos.2019.94103
Muñiz-Diaz R, Gutiérrez de la Rosa SY, GutiérrezCoronado Ó, Patakfalvi R (2022) Biogenicsynthes is of platinum nanoparticles. Chem Pap 76(5):2573–2594. https://doi.org/10.1007/s11696-021-01970-8
An X, Pei Y, Chen S, Li S, Hu X, Chen G, Lin B, Wang H (2016) Three new but enolides from the fungus Aspergillus sp. CBS-P-2. Molecules 21(10):1361. https://doi.org/10.3390/molecules21101361
Saravanakumar K, Sriram B, Sathiyaseelan A, Hu X, Mariadoss AVA, MubarakAli D, Wang MH (2021) Molecular identification, volatile metabolites profiling, and bioactivities of an indigenous endophytic fungus (Diaporthe sp.). Process Biochem 102:72–81. https://doi.org/10.1016/j.procbio.2020.12.002
Starzyńska-Janiszewska A, Duliński R, Stodolak B (2020) Fermentation with edible Rhizopus strains to enhance the bioactive potential of hull-less pumpkin oil cake. Molecules 25(24):5782. https://doi.org/10.3390/molecules25245782
Barus T, Titarsole NN, Mulyono N, Prasasty VD (2019) Tempeh antioxidant activity using DPPH method: effects of fermentation, processing, and microorganisms. Journal of Food Engineering and Technology 8(2):75–80. https://doi.org/10.32732/jfet.2019.8.2.75
Sugiharto S, Yudiarti T, Isroli I (2016) Assay of antioxidant potential of two filamentous fungi isolated from the Indonesian fermented dried cassava. Antioxidants 5(1):6. https://doi.org/10.3390/antiox5010006
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 86(11):807–815. https://doi.org/10.1016/j.biochi.2004.09.017
Xie D, Jackson EN, Zhu Q (2015) Sustainable source of omega-3 eicosapentaenoic acid from metabolically engineered Yarrowia lipolytica: from fundamental research to commercial production. Appl Microbiol Biotechnol 99(4):1599–1610. https://doi.org/10.1007/s00253-014-6318-y
Fu B, Zhang J, Fan J, Wang J, Liu H (2012) Control of C/N ratio for butyric acid production from textile wastewater sludge by anaerobic digestion. Water Sci Technol 65(5):883–889. https://doi.org/10.2166/wst.2012.919
Maiti S, Brar SK, Verma M, Soccol CR, Maiti DC (2016) Chapter 7 - butyric acid: a platform chemical for biofuel and high-value biochemical. In: Brar SK, Sarma SJ, Pakshirajan K (eds), Platform Chemical Biorefinery, Elsevier, pp 119–132.
Martinez-Medina GA, Chávez-González ML, Verma DK, Prado-Barragán LA, Martínez-Hernández JL, Flores-Gallegos AC, Thakur M, Srivastav PP, Aguilar CN (2021) Bio-funcional components in mushrooms, a health opportunity: ergothionine and huitlacohe as recent trends. J Funct Foods 77:104326. https://doi.org/10.1016/J.JFF.2020.104326
Sande D, de Oliveira GP, Moura MAF, Martins BA, Lima MTNS, Takahashi JA (2019) Edible mushrooms as a ubiquitous source of essential fattyacids. Food Res Int 125:108524. https://doi.org/10.1016/J.FOODRES.2019.108524
Yılmaz N, Solmaz M, Türkekul İ, Elmastaş M (2006) Fatty acid composition in some wild edible mushrooms growing in them iddle Black Searegion of Turkey. Food Chem 99(1):168–174. https://doi.org/10.1016/j.foodchem.2005.08.017
Doǧan HH, Akbaş G (2013) Biological activity and fatty acid composition of Caesar’s mushroom. Pharm Biol 51(7):863–871. https://doi.org/10.3109/13880209.2013.768272
Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, De losReyes-Gavilán CG, Salazar N (2016) Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 7(185). https://doi.org/10.3389/fmicb.2016.00185
Lapiere A, Richard ML (2022) Bacterial-fungal metabolic interactions within the microbiota and their potential relevance in human health and disease: a short review. Gut Microbes 14(1). https://doi.org/10.1080/19490976.2022.2105610
Salsinha AS, Machado M, Rodríguez-Alcalá LM, Gomes AM, Pintado M (2023) Chemistry, biochemistry, and biological properties. In: Pintado M et al (eds) Bioactive Lipids, 1st edn. AcademicPress, Porto, pp 1–35. https://doi.org/10.1016/b978-0-12-824043-4.00014-2
Ribeiro B, Guedes de Pinho P, Andrade PB, Baptista P, Valentão P (2009) Fatty acid composition of wild e dible mushrooms species: a comparative study. Microchem J 93(1):29–35. https://doi.org/10.1016/J.MICROC.2009.04.005
Chowdhury SK, Dutta T, Chattopadhyay AP, Ghosh NN, Chowdhury S, Mandal V (2021) Isolation of antimicrobial Tridecanoic acid from Bacillus sp. LBF-01 and its potentialization through silver nanoparticles synthesis: a combined experimental and theoretical studies. J Nanostructure Chem 11(4):573–587. https://doi.org/10.1007/s40097-020-00385-3
Venn-Watson S, Lumpkin R, Dennis EA (2020) Efficacy of dietary odd-chain saturated fatty acid penta decanoic acid parallels broad associated health benefits in humans: could it be essential? Sci Rep 10 (1). https://doi.org/10.1038/s41598-020-64960-y
Chen WS, Strik DPBTB, Buisman CJN, Kroeze C (2017) Production of caproic acid from mixed organic waste: an environmental life cycle perspective. Environ Sci Technol 51(12):7159–7168. https://doi.org/10.1021/acs.est.6b06220
Mungali M, Sharma N, Gauri (2021) Caprylic/caprictriglyceride. In: Belwal T, Nabavi SM, Nabavi SF, Dehpour AR, Shirooie S (eds) Naturally occurring chemicals against Alzheimer’s disease, London, pp 139–146. https://doi.org/10.1016/B978-0-12-819212-2.00011-6
Roopashree PG, Shetty SS, SuchethaKumari NS (2021) Effect of medium chain fatty acid in human health and disease. Journal of Functional Foods 87:104724. https://doi.org/10.1016/J.JFF.2021.104724
Narayanan A, Baskaran SA, Amalaradjou MAR, Venkitanarayanan K (2015) Anticarcinogenic properties of medium chain fatty acids on human colorectal, skin and breast cancer cells in vitro. Int J Mol Sci 16(3):5014–5027. https://doi.org/10.3390/ijms16035014
Fauser JK, Matthews GM, Cummins AG, Howarth GS (2014) Induction of apoptosis by the medium-chain length fatty acid lauric acid in colon cancer cells due to induction of oxidative stress. Chemotherapy 59(3):214–224. https://doi.org/10.1159/000356067
Sheela DL, Narayanankutty A, Nazeem PA, Raghavamenon AC, Muthangaparambil SR (2019) Lauric acid induce cell death in colon cancer cells mediated by the epidermal growth factor receptor down regulation: an in silico and in vitro study. Hum Exp Toxicol 38(7):753–761. https://doi.org/10.1177/0960327119839185
Lappano R, Sebastiani A, Cirillo F, Rigiracciolo DC, Galli GR, Curcio R, Malaguarnera R, Belfiore A, Cappello AR, Maggiolini M (2017) The lauric acid-activated signaling prompts apoptosis in cancer cells. Cell Death Discov 3(1). https://doi.org/10.1038/cddiscovery.2017.63
Kim YG, Lee JH, Park S, Kim S, Lee J (2022) Inhibition of polymicrobial biofilm formation by saw palmetto oil, lauric acid and myristic acid. Microb Biotechnol 15(2):590–602. https://doi.org/10.1111/1751-7915.13864
Casillas-Vargas G, Ocasio-Malavé C, Medina S, Morales-Guzmán C, Del Valle RG, Carballeira NM, Sanabria-Ríos DJ (2021) Antibacterial fatty acids: an update of possible mechanisms of action and implications in thedevelopment of thenext-generation of antibacterial agents. Prog Lipid Res 82:101093. https://doi.org/10.1016/J.PLIPRES.2021.101093
Sakai H, Matsumoto K, Urano T, Sakane F (2022) Myristic acid selectively augments β-tubulin levels in C2C12 myotubes via diacylglycerolkinase δ. FEBS Open Bio 12(10). https://doi.org/10.1002/2211-5463.13466
Cellat K, Beyhan B, Güngör C, Konuklu Y, Karahan O, Dündar C, Paksoy H (2015) Thermal enhancement of concrete by adding bio-based fatty acids as phase change materials. Energy Build 106:156–163. https://doi.org/10.1016/J.ENBUILD.2015.05.035
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
GB: investigation, writing—original draft; ÜDG: supervision, conceptualization, methodology, visualization, investigation, writing—original draft, writing—review and editing; BET: investigation, writing—original draft, writing—review and editing; FT: investigation, methodology, writing—original draft, writing—review and editing; RG: investigation, methodology, visualization, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Ethics approval was not required for this study.
Consent to participate
All authors are informed and provided consent for this submission.
Consent for publication
All authors have approved the manuscript and declare that this is an original contribution and none of the material in this paper is under consideration for publication elsewhere.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bayazıt, G., Gül, Ü.D., Taştan, B.E. et al. Exploring the biotechnological prospects of a recently discovered fungus isolated from marine mucilage. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05945-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05945-z