Abstract
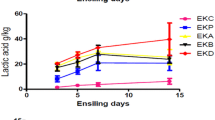
The objective of this study was to characterize the probiotic lactic acid bacteria to improve the quality of silage. A total of 42 LAB isolates were isolated from the date syrup, and the potent strains were characterized as Lactobacillus casei LC09 and L. lactis LL25. These two strains showed the ability to survive at low pH, stimulate gastric conditions, and be sensitive to most of the prominent antibiotics. LAB strains have adhesion ability (p < 0.001) on the surface of the HT-29 cell lines and γ-hemolytic type, and no zones developed around the blood agar medium. The cell-free extract of the selected LAB showed promising DPPH and ferric reducing power activity. Among the selected fungi, LC09 showed the maximum zone of inhibition against Fusarium oxysporum (26 ± 1 mm), Aspergillus niger (27 ± 2 mm), and Fusarium graminearum (26 ± 1 mm). The strain LC09 showed maximum activity against F. graminearum (29 ± 1 mm) and F. oxysporum (24 ± 2 mm). Acetic acid and lactic acid production are the prominent end products determined from the cell free extract of both LAB (p < 0.001). Lactic acid production was 5.3 ± 0.32 g/L and 4.7 ± 0.28 g/L for strains LC09 and LL25, whereas, acetic acid level was 0.39 ± 0.03 g/L and 0.27 ± 0.47 g/L for these LAB. Quail bush and date wastes were used for the preparation of silage, and LAB strains were inoculated and treated for 30 days. In vitro silage analysis showed that quail bush-date waste inoculated with LC09 and LL25 showed a decreased pH level at the end of fermentation (after 30 days) (p < 0.001). Lactic acid and acetic acid levels were increased in the experimental silage (p < 0.001). Thus, ensiled quail bush-date waste could promote silage quality, improve fibre digestion and reduce the growth of pathogenic bacteria and fungal strains.



Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Soundharrajan I, Kim D, Kuppusamy P et al (2019) Probiotic and Triticale silage fermentation potential of Pediococcus pentosaceus and Lactobacillus brevis and their impacts on pathogenic bacteria. Microorganisms 7:318. https://doi.org/10.3390/microorganisms7090318
Ilavenil S, Vijayakumar M, Kim DH et al (2016) Assessment of probiotic, antifungal and cholesterol lowering properties of Pediococcus pentosaceus KCC-23 isolated from Italian ryegrass. J Sci Food Agric 96(2):593–601. https://doi.org/10.1002/jsfa.7128
Lahtinen S, Ouwehand AC, Salminen S, von Wright A (eds) (2011) Lactic acid bacteria: microbiological and functional aspects. Crc Press
Arasu MV, Kim DH, Kim PI et al (2014) In vitro antifungal, probiotic and antioxidant properties of novel Lactobacillus plantarum K46 isolated from fermented sesame leaf. Ann Microbiol 64:1333–1346. https://doi.org/10.1007/s13213-013-0777-8
Roth APDTP, Reis RA, Siqueira GR et al (2010) Sugarcane silage production treated with additives at different times post burning. R Bras Zootec 39:88–96. https://doi.org/10.1590/S1516-35982010000100012
Pedroso ADF, Nussio LG, Loures DRS et al (2008) Fermentation, losses, and aerobic stability of sugarcane silages treated with chemical or bacterial additives. Scientia Agricola 65:589–594. https://doi.org/10.1590/S0103-90162008000600004
Ávila CLS, Schwan RF, Pinto JC, Carvalho BF (2011) Potential use of native microorganisms strains of forage for silage production. In: II Symposium on Forage Quality and Conservation. Zopollatto M, Daniel LLP, Nussio LG, Sa Neto A (eds) Fundacao de Estudos Agrarios Luiz de Queiroz (FEALQ), Piracicaba, Brazil, pp 25–44
Ávila CLDS, Valeriano AR, Pinto JC et al (2010) Chemical and microbiological characteristics of sugar cane silages treated with microbial inoculants. Braz J Ani Sci 39:25–32. https://doi.org/10.1590/S1516-35982010000100004
Siedler S, Rau MH, Bidstrup S et al (2020) Competitive exclusion is a major bioprotective mechanism of lactobacilli against fungal spoilage in fermented milk products. Appl Env Microbiol 86(7):e02312-e2319. https://doi.org/10.1128/AEM.02312-19
Axel C, Zannini E, Arendt EK (2017) Mold spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Cri Rev Food Sci Nut 57(16):3528–3542. https://doi.org/10.1080/10408398.2016.1147417
Parappilly SJ, Idicula DV, Chandran A, Mathil Radhakrishnan K et al (2021) Antifungal activity of human gut lactic acid bacteria against aflatoxigenic Aspergillus flavus MTCC 2798 and their potential application as food biopreservative. J Food Safety 41(6):e12942. https://doi.org/10.1111/jfs.12942
Crowley S, Mahony J, van Sinderen D (2013) Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Tren Food Sci Technol 33(2):93–109. https://doi.org/10.1016/j.tifs.2013.07.004
Contreras-Govea FE, Muck RE, Broderick GA, Weimer PJ (2013) Lactobacillus plantarum effects on silage fermentation and in vitro microbial yield. Ani Feed Sci Technol 179(1–4):61–68. https://doi.org/10.1016/j.anifeedsci.2012.11.008
Neres MA, Zambom MA, Fernandes T et al (2013) Microbiological profile and aerobic stability of Tifton 85 bermudagrass silage with different additives. R Bras Zootec 42:381–387. https://doi.org/10.1590/S1516-35982013000600001
Soundharrajan I, Kim DH, Srisesharam S et al (2017) Application of customised bacterial inoculants for grass haylage production and its effectiveness on nutrient composition and fermentation quality of haylage. 3 Biotech 7:1–9. https://doi.org/10.1007/s13205-017-0965-5
Valan Arasu M, Jung MW, Kim DH et al (2015) Identification and phylogenetic characterization of novel Lactobacillus plantarum species and their metabolite profiles in grass silage. Ann Microbiol 65:15–25. https://doi.org/10.1007/s13213-014-0830-2
Oliveira AS, Weinberg ZG, Ogunade IM et al (2017) Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J Dairy Sci 100(6):4587–4603. https://doi.org/10.3168/jds.2016-11815
Muck RE, Nadeau EMG, McAllister TA et al (2018) Silage review: Recent advances and future uses of silage additives. J Dairy Sci 101(5):3980–4000. https://doi.org/10.3168/jds.2017-13839
Al-Turki TA, Omer S, Ghafoor A (2000) A synopsis of the genus Atriplex L.(Chenopodiaceae) in Saudi Arabia. Feddes Repertorium 111(5–6):261–293. https://doi.org/10.1002/fedr.20001110503
Vasta V, Nudda A, Cannas A et al (2008) Alternative feed resources and their effects on the quality of meat and milk from small ruminants. Ani Feed Sci Technol 147(1–3):223–246. https://doi.org/10.1016/j.anifeedsci.2007.09.020
Oh NS, Lee JY, Oh S et al (2016) Improved functionality of fermented milk is mediated by the synbiotic interaction between Cudrania tricuspidata leaf extract and Lactobacillus gasseri strains. Appl Microbiol Biotechnol 100(13):5919–5932. https://doi.org/10.1007/s00253-016-7414-y
Tadesse BT, Tesfaye A, Muleta D et al (2018) Isolation and molecular identification of lactic acid bacteria using 16s rRNA genes from fermented Teff (Eragrostis tef (Zucc.)) dough. Int J Food Sci 2018:8510620. https://doi.org/10.1155/2018/8510620
Leite AM, Miguel MAL, Peixoto RS et al (2015) Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J Dairy Sci 98(6):3622–3632. https://doi.org/10.3168/jds.2014-9265
Kim JH, Baik SH (2019) Probiotic properties of Lactobacillus strains with high cinnamoyl esterase activity isolated from jeot-gal, a high-salt fermented seafood. Ann Microbiol 69:407–417. https://doi.org/10.1007/s13213-018-1424-1
Thaipong K, Boonprakob U, Crosby K et al (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal 19(6–7):669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Bolsen KK, Lin C, Brent BE et al (1992) Effect of silage additives on the microbial succession and fermentation process of alfalfa and corn silages. J Dairy Sci 75(11):3066–3083. https://doi.org/10.3168/jds.S0022-0302(92)78070-9
Varghese LK, Rizvi AF, Gupta AK (2013) Isolation, screening and biochemical characterization of pectinolytic microorganism from soil sample of Raipur city. J Biol Chem Res 30(2):636–643
Thiex N, Novotny L, Crawford A (2012) Determination of ash in animal feed: AOAC official method 942.05 revisited. J AOAC Int 95(5):1392–1397. https://doi.org/10.5740/jaoacint.12-129
Horwitz W, Latimer GW (2000) Official methods of analysis of AOAC International (vol 1, p 17). Gaithersburg: AOAC international
Pringsulaka O, Rueangyotchanthana K, Suwannasai N et al (2015) In vitro screening of lactic acid bacteria for multi-strain probiotics. Livest Sci 174:66–73. https://doi.org/10.1016/j.livsci.2015.01.016
Guo H, Pan L, Li L et al (2017) Characterization of antibiotic resistance genes from Lactobacillus isolated from traditional dairy products. J Food Sci 82(3):724–730. https://doi.org/10.1111/1750-3841.13645
Gad GFM, Abdel-Hamid AM, Farag ZSH (2014) Antibiotic resistance in lactic acid bacteria isolated from some pharmaceutical and dairy products. Braz J Microbiol 45:25–33. https://doi.org/10.1590/S1517-83822014000100005
Gao Y, Li D, Liu S, Liu Y (2012) Probiotic potential of L. sake C2 isolated from traditional Chinese fermented cabbage. Eur Food Res Technol 234:45–51. https://doi.org/10.1007/s00217-011-1608-4
Nawaz AN, Jagadeesh KS, Krishnaraj PU (2017) Isolation and screening of lactic acid bacteria for acidic pH and bile tolerance. Int J Curr Microbiol Appl Sci 6(7):3975–3980
Archer AC, Halami PM (2015) Probiotic attributes of Lactobacillus fermentum isolated from human feces and dairy products. Appl Microbiol Biotechnol 99:8113–8123. https://doi.org/10.1007/s00253-015-6679-x
Shokryazdan P, Sieo CC, Kalavathy R et al (2014) Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. BioMed Res Int 2014, Article 927268. https://doi.org/10.1155/2014/927268
Muryany I, Lian HH, Ina-Salwany ARG et al (2018) Adhesion ability and cytotoxic evaluation of Lactobacillus strains isolated from Malaysian fermented fish (pekasam) on Ht-29 and Ccd-18Co intestinal cells. Sains Malays 47:2391–2399. https://doi.org/10.17576/jsm-2018-4710-15
Padmavathi T, Bhargavi R, Priyanka PR et al (2018) Screening of potential probiotic lactic acid bacteria and production of amylase and its partial purification. J Genet Eng Biotechnol 16(2):357–362. https://doi.org/10.1016/j.jgeb.2018.03.005
Deng F, Chen Y, Sun T et al (2021) Antimicrobial resistance, virulence characteristics and genotypes of Bacillus spp. from probiotic products of diverse origins. Food Res Int 139:109949. https://doi.org/10.1016/j.foodres.2020.109949
Muñoz R, de Las Rivas B, de Felipe FL et al (2017) Biotransformation of phenolics by Lactobacillus plantarum in fermented foods. In: Fermented foods in health and disease prevention (pp 63–83). Academic Press. https://doi.org/10.1016/B978-0-12-802309-9.00004-2
Son SH, Jeon HL, Jeon EB et al (2017) Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT Food Sci Technol 85:181–186. https://doi.org/10.1016/j.lwt.2017.07.018
Deepa N, Rakesh S, Sreenivasa MY (2018) Morphological, pathological and mycotoxicological variations among Fusarium verticillioides isolated from cereals. 3 Biotech 8:1–10. https://doi.org/10.1007/s13205-018-1136-z
Bangar SP, Suri S, Trif M, Ozogul F (2022) Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci 46:101615. https://doi.org/10.1016/j.fbio.2022.101615
Guimarães A, Venancio A, Abrunhosa L (2018) Antifungal effect of organic acids from lactic acid bacteria on Penicillium nordicum. Food Addit Contam: Part A 35(9):1803–1818. https://doi.org/10.1080/19440049.2018.1500718
Vidhyasagar V, Saraniya A, Jeevaratnam K (2013) Identification of pectin degrading lactic acid bacteria from fermented food sources. Int J Adv Life Sci 6(1):8–12
Chatterjee E, Manuel GAS, Hassan S (2016) Effect of fruit pectin on growth of lactic acid bacteria. J Probio Health 4(2):1000147
Garcia C, Guerin M, Souidi K, Remize F (2020) Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 6(1):8. https://doi.org/10.3390/beverages6010008
Drouin P, Tremblay J, Chaucheyras-Durand F (2019) Dynamic succession of microbiota during ensiling of whole plant corn following inoculation with Lactobacillus buchneri and Lactobacillus hilgardii alone or in combination. Microorganisms 7(12):595. https://doi.org/10.3390/microorganisms7120595
Silva VP, Pereira OG, Leandro ES et al (2020) Selection of lactic acid bacteria from alfalfa silage and its effects as inoculant on silage fermentation. Agriculture 10(11):518. https://doi.org/10.3390/agriculture10110518
Li J, Wang W, Chen S et al (2021) Effect of lactic acid bacteria on the fermentation quality and mycotoxins concentrations of corn silage infested with mycotoxigenic fungi. Toxins 13(10):699. https://doi.org/10.3390/toxins13100699
Weinberg ZG, Ashbell G, Hen Y, Azrieli A (1993) The effect of applying lactic acid bacteria at ensiling on the aerobic stability of silages. J Appl Microbiol 75(6):512–518. https://doi.org/10.1111/j.1365-2672.1993.tb01588.x
Filya I (2003) The effect of Lactobacillus buchneri, with or without homofermentative lactic acid bacteria, on the fermentation, aerobic stability and ruminal degradability of wheat, sorghum and maize silages. J Appl Microbiol 95(5):1080–1086. https://doi.org/10.1046/j.1365-2672.2003.02081.x
Joo YH, Kim DH, Paradhipta DH et al (2018) Effect of microbial inoculants on fermentation quality and aerobic stability of sweet potato vine silage. Asian-Australasian J Anim Sci 31(12):1897. https://doi.org/10.5713/ajas.18.0264
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R190), King Saud University, Riyadh, Saudi Arabia
Author information
Authors and Affiliations
Contributions
DAA: methodology, formal analysis, TAS: methodology, data curation, review, MSE: validation, review, editing, project administration, P.V: methodology, investigation, original draft.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alfarraj, D.A., Sathya, T.A., Elshikh, M.S. et al. Nutritive value and aerobic stability of whole quail bush and date waste silage ensiled at different compositions and the role of hetero-fermentative lactic acid bacteria. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05744-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05744-6