Abstract
Pectic enzyme groups, particularly polygalacturonase and pectin lyase, are vital components of a high-value microbial enzyme category widely employed in applications within the fruit juice and wine industries. The exploration of alternative carbon and nitrogen sources remains crucial for enhancing enzyme production while reducing costs. This study evaluates the impact of carbon (black carrot pomace) and nitrogen (pea protein) loading on fermentable sugar content, protein content, and enzyme activities during both batch and fed-batch cultivation. Additionally, three distinct valorization techniques—thermal (steam), thermochemical (steam assisted with dilute acid), and microwave-assisted with dilute acid pretreatments—were assessed for their effectiveness in hydrolyzing black carrot pomace as a carbon source. The findings indicate that microwave-assisted dilute acid, coupled with enzymatic saccharification, resulted in the highest fermentable sugar production (0.493 g/g), achieving an 87.3% conversion yield. Pea protein demonstrated more favorable outcomes with the highest polygalacturonase activity (20.50 ± 0.52 U/L) and pectin lyase activity (46.44 ± 3.45 U/L) compared to whey protein and yeast extract used as nitrogen sources. Meanwhile, the highest polygalacturonase and pectin lyase activity, along with the highest total protein content (52.25 ± 0.06 mg/L), was recorded under the same culture conditions, reaching 164.34 ± 2.26 and 188.22 ± 1.72 U/L, respectively, after 72 h, representing approximately 1.18- and 1.34-fold increases from the batch system. Consequently, these results prove that fed-batch cultivation, utilizing black carrot pomace hydrolyzate as a feeding substrate and pea protein as a nitrogen source, significantly increases polygalacturonase and pectin lyase activity compared to batch cultivation.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Pectinase production accounts for a significant share (25%) of the total enzyme production market in the world [1]. Commercial pectinase consists of three enzymatic activities, with polygalacturonase (PG) and pectin lyase (PNL) being the most prominent. PG and PNL play crucial role in depectinization processes, such as grape must depectinization and apple or pear juice processing [2]. In the literature, fungal organisms have been predominantly utilized for the production of pectinolytic enzymes, whereas research on bacterial pectinases is comparatively scarce. However, fungal-derived pectinases frequently suffer from restricted applicability due to their narrow pH range [3]. The utilization of a biological process for the production of these crucial enzymes from inexpensive substrates, coupled with a cost-effective fermentation approach, remains highly sought after [4].
Enzymes are typically produced through batch, fed batch, or continuous fermentation methods. Fed-batch and continuous methods are preferred for longer incubation periods due to their higher productivity compared to batch fermentation. Fed-batch fermentation is particularly advantageous when high nutrient concentrations, which may inhibit cell productivity, are involved. It allows for precise control of nutrient feeding, optimizing product yield [5]. Submerged fermentation is widely used for enzyme production; however, substrate inhibition and eventual decrease in product concentration often occur under high initial substrate concentration during batch fermentation. Therefore, it leads to the development of fed batch process which eliminates substrate inhibition and enhancing productivity. Fed batch fermentation offers the advantage of adjusting substrate concentrations in the culture broth for optimal cell growth and production [6].
The cost of enzyme production is also significantly influenced by the fermentation medium. Utilizing food waste as a substrate is gaining interest to reduce production costs. Black carrot pomace (BCP), an industrial by-product of fruit juice processing, is an abundant source of enzymes in Turkey [7]. BCP utilization as a carbon source for enzyme production has been shown to increase enzyme activity while reducing production costs [8]. In Turkey, according to Turkish Statistics Institution (TUIK)’s data, in 2017 over 120 kilotons of black carrot was produced. Black carrot is generally used in the production of a traditional fermented beverage called “shalgam” in Turkey. Around 2700 and 6900 tons of black carrot pomace (BCP), an industrial by-product of fruit juice processing in Turkey, was produced in 2009 and 2010, respectively [9]. Therefore, black carrot pomace (BCP) is used as a substrate to produce high-value-added products such as enzymes, aroma compounds, ethanol, and organic acids both economically and ecologically. The utilization of BCP as carbon sources in enzyme blend production has increased enzyme activity with reduction of the production cost [10].
Kaur and Gupta [11] conducted a study on the production of pectin-degrading enzymes (pectinase and pectin lyase) using solid-state fermentation by Bacillus subtilis SAV-21. They employed a combination of orange peel and coconut fiber as substrates, maintaining a temperature of 35 °C and a pH of 4.0. In a related investigation, Amande et al. [12] documented the solid-state fermentation process for producing polygalacturonase (PG) and pectin lyase (PL) using various substrates, including beans testa, mango peels, and plantain peels. The study utilized fungal strains such as Aspergillus tamarii, Aspergillus terreus, Aspergillus piperis, Aspergillus parasiticus, and Mucor piriformis for enzyme production. El Enshasy et al. [13] investigated the pectinase production potential of a novel strain, Aspergillus niger NRC1ami, utilizing a statistically optimized medium. Medium optimization led to a remarkable enhancement in pectinase production, increasing yields by approximately 2.8-fold. Furthermore, the enzyme yield underwent an additional four-fold improvement upon transitioning the process from shake flasks to bioreactors through fed-batch cultivation with continuous carbon source feeding. Most previous studies have focused on enzyme production using submerged or solid-state fermentation. However, this study investigates the influence of nitrogen sources in batch and fed-batch cultivation on the co-production of polygalacturonase and pectin lyase for the first time. In the present study, a novel approach was applied that utilized BCP as a carbon source and pea protein as a nitrogen source through batch and fed-batch fermentation. The objectives of this study included (1) assessing the potential of black carrot pomace as substrate; (2) determining the significance of nitrogen sources during enzyme synthesis; and (3) establishing correlations between fermentable sugar content, biomass, protein content, and co-production of PG and PNL under optimal conditions in fed-batch fermentation.
2 Materials and methods
2.1 Raw material
Black carrot pomace (BCP) was obtained from Goknur Foodstuff Company in Nigde, Turkey, and dried at 70 °C in an oven for 6 h upon arrival. Then, the dried BCP was stored inside plastic bags and kept at room temperature until use. Viscozyme L, phenol, and 3,5-dinitrosalicylic acid (DNS) were purchased from Sigma Aldrich, Taufkirchen, Germany. The other chemicals such as sulfuric acid (H2SO4), sodium hydroxide (NaOH), and sodium sulfite were purchased from Merck, Deisenhofen, Germany.
2.2 Microorganism and inoculum preparation
The strain Bacillus subtilis ATCC 6633 was provided by the Hıfzısıhha Institute, Halk Saglıgı Laboratory, Ankara, Turkey. Stock cultures of B. subtilis ATCC 6633 were activated on nutrient agar at 37 °C and maintained at 4 °C. The growth medium for B. subtilis ATCC 6633 consists of pectin (2.0 g/L), yeast extract (1.0 g/L), glucose (10.0 g/L), K2HPO4 (0.4 g/L), KH2PO4 (0.2 g/L), and MgSO4.7H2O (0.4 g/L). The growth medium was incubated at 35 °C for 14 h with 120 rpm [14]. Stock cultures of B. subtilis ATCC 6633 were prepared with 40% glycerol-distilled water and stored at − 80 °C.
2.3 Preparation of BCP hydrolyzate as a carbon source
Three pretreatment methods, thermal (T), thermo-chemical (TC), and microwave-assisted dilute acid (MW-DA), were employed to evaluate the fermentable sugar content. In thermal pretreatment, 5 g of dried BCP was mixed with 100 mL of water and autoclaved at 121 °C for 15 min. TC pretreatment was conducted in an autoclave at 121 °C for 15 min with 2% H2SO4 as the acid concentration with 1:20 solid/liquid ratio [15]. MW-DA pretreatment utilized a domestic microwave oven (Samsung) as a microwave source. Statistical analyses were conducted to evaluate MW-DA pretreatment in terms of FSC as a function of power and pretreatment time. The orthogonal arrays for control factors are shown in Table 1. The L9 (2^3) three-level Taguchi design with 2 factors and 3 levels was generated by MINITAB 16.0 (Minitab Inc., State College, PA, USA). The levels of two variables were determined through preliminary experiments. The Taguchi design was constructed by using two predictors with three levels, in each of which, power (P: 300, 600, and 850 W) and pretreatment time (t: 30, 60, and 90 s) are used (Table 1). The quality performance was evaluated at “nominal the best.”
After pretreatment, the liquid was separated from solid residue by centrifugation at 10,000 rpm for 10 min. After centrifugation, the pH of the hydrolysate samples was adjusted to 5.0 with 10% NaOH solution. Subsequently, the treated samples were hydrolyzed using Viscozyme L (sigma-Aldrich, Germany) at 100 U/g for 24 h according to the previous study. One Viscozyme L unit was defined as the amount of enzyme that liberates 1 μmol of reducing sugars equivalent to glucose per min at 50 °C and pH 5.0 for 30 min. After the enzymatic hydrolysis, samples were heated to 100 °C for 15 min to inactivate the enzymes [16]. Fermentable sugar content was determined using the DNS method [17].
The fermentable sugar content (FSC) and energy consumption were calculated as follows [18]:
where DF denotes the dilution factor.
Energy consumption (kJ/g FSC) = microwave or autoclave power (kW) * time (s)/FSC in pretreatment (g).
2.4 Preparation of nitrogen sources
Nitrogen sources were evaluated similarly by replacing yeast extract (5 g/L) (Merck, Germany) with other nitrogen sources such as soluble pea (NUTRALYS® S, Roquette, France) and whey protein powder (Hardline).
2.5 Co-production of polygalacturonase and pectin lyase in a submerged cultivation system: batch and fed-batch fermentation
Submerged cultivation was conducted in two stages, batch and fed-batch fermentation, using 500-mL Erlenmayer flasks (working volume 200 mL). The fermentation medium contained BCP hydrolysate (instead of glucose), pea protein powder (instead of yeast extract) at a concentration of 30 g/L, K2HPO4 (0.4 g/L), and MgSO4.7H2O (1 g/L). Before inoculation, the medium was autoclaved at 121 °C for 15 min. Then, the fermentation medium was inoculated with 1 mL of bacterial culture containing 3 * 107 CFU/mL and incubated at 120 rpm at 35 °C for 96 h. Fed-batch experiments were conducted by the addition of 2.5, 5, 10, and 15 mL of BCP hydrolyzate to the fermentation medium after 54 h. Changes in enzyme production, FSC, cell density, and protein content were monitored with samples taken every 12 h. Crude enzyme activity was determined after centrifugation at 10,000 rpm for 10 min.
2.5.1 Effect of nitrogen sources on enzyme production
Various organic nitrogen sources, including yeast extract, whey protein, and pea protein, were investigated to determine the most effective nitrogen source for co-production of PG and PNL enzyme activity. Following batch fermentation, the co-production of PG and PNL was assessed.
2.5.2 Effect of incubation time on enzyme production
The samples were collected at 12-h intervals over a 96 h fermentation period and subsequently analyzed to evaluate pectinase production.
2.5.3 Effect of pea protein loading on enzyme production
While maintaining constant conditions such as BCP hydrolyzate, pH, incubation time, temperature, inoculum volume, magnesium sulfate, and dipotassium hydrogen phosphate, varying amounts of pea protein (0.25, 0.75, 1.5, and 2 g) were added during fermentation.
2.5.4 Effect of BCP hydrolyzate feeding on enzyme production
A comparative analysis was conducted between a fed-batch and batch process at four different substrate concentrations (2.5, 5, 10, and 15% BCP hydrolyzate) to evaluate their impact on co-production of PG and PNL.
2.6 Analytical analysis
The following analyses were performed on the samples obtained as a result of fermentation: proximate analysis of BCP, fermentable sugar content, optical density, protein content, and enzyme activities.
2.6.1 Proximate analysis
The moisture, ash, fat, and protein contents of black carrot pomace (BCP) were determined. Moisture content was assessed by drying the samples at 105 °C until a constant weight was achieved. Ash content was determined by burning dried samples in an oven at 550 °C until constant weight was reached [19]. For fat content, oven dried sample was weighed into thimbles and placed in a Soxhlet apparatus filled with 300 mL of hexane and heated for 6 h. The hexane and oil mixture was dried in oven [20]. The total protein content of the BCP was determined by the method of Lowry et al. [21]. The untreated and treated BCP samples were subjected to compositional analysis by following two stage acid hydrolysis according to the National Renewable Energy Laboratory (NREL) protocol [22]. The identification and quantification of sugars and inhibitors such as hydroxymethyl furfural (HMF) were performed in HPLC system (Prostar, Varian, CA, USA) equipped with an RI detector for HMF and sugars using Metacarb 87H column (300 mm × 7.8 mm, Varian, S/N: 05517112, Varian) and the operation conditions were 35 °C with 0.08 N H2SO4 as the eluent flowing at 0.5 mL/min [23].
2.6.2 Cell density determination
The cell density was determined by measuring the turbidity at 600 nm. Each analysis was duplicated for each replicate, and mean values were reported.
2.6.3 Fermentable sugar content
Fermentable sugar content (FSC) was estimated using the DNS method [17]. A standard curve was prepared using D-glucose solutions at 0.15–1.0 g/L.
2.6.4 Protein content
The total protein content of the samples was determined according to the method of Lowry et al. [21], using bovine serum albumin (BSA) as the protein standard. The absorbance of each sample was measured at 750 nm against a blank.
2.6.5 Enzyme activity assays
DNS method was employed to determine the activity of PG enzyme [17] and thiobarbutyric acid (TBA) method was used for PNL activity [24]. Polygalacturonase activity (PG) was determined by incubating samples at 50 °C and pH 7.0 for 30 min according to the method of Kapoor et al. [14] at 575 nm with galacturonic acid monohydrate as the standard. One unit of polygalacturonase activity was defined as the amount of enzyme that produced 1 μmole of D-galacturonic acid per minute under assay conditions. Pectinlyase (PNL) activity was assessed by measuring the absorption of unsaturated uronic ester derivatives, as described by Nedjma et al. [24]. To initiate the assay, 0.25 mL of enzyme solution was added to 0.25 mL of substrate solution (1.5% pectin, ED 93%) and incubated at 37 °C for 10 min. Following this incubation period, 50 μL of 1 N NaOH was added to the 0.5 mL mixture, and the resulting solution was incubated in a water bath at 80 °C for 5 min, resulting in the appearance of a yellow coloration. Subsequently, 0.6 mL of 1 N HCl was added to acidify the medium, causing the yellow color to disappear. After shaking the solution, 0.5 mL of 0.04 M thiobarbutyric acid was added. The tube was then incubated for an additional 5 min at 80 °C. Finally, the solution was briefly cooled in an ice-water bath before measuring the absorbance at 550 nm. A control was prepared by adding 1% NaCl instead of enzyme solution. One unit of pectin lyase (PNL) activity was defined as the amount of enzyme that produced an increase of one unit of absorbance at 550 nm per minute [24].
2.7 Statistical analysis
Statistical significance between groups was assessed using analysis of variance (ANOVA). Analysis of variance (ANOVA) and regression models were performed at a 95% confidence interval (p < 0.05) to identify the significant terms of the predictive model. The pairwise comparisons were made by Tukey’s test with a significance level of 0.05. All the experiments were conducted in triplicate.
3 Results and discussion
3.1 Composition of raw material
BCP contained 5.17 ± 0.07% ash, 8.63 ± 0.19% moisture, 4.83 ± 0.23% protein, 1.15 ± 0.01% fat (dry basis), and 0.565 ± 0.001 g fermentable sugar/g dry biomass, showing its potential as a source of sugars such as glucose, xylose, and arabinose. These results align with those reported by Elik [25], who investigated hot-air-assisted radio frequency drying of BCP.
3.2 Effect of different pretreatment strategies on FSC
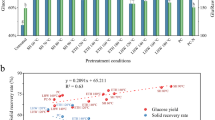
The effectiveness of thermal, thermo-chemical, and microwave pretreatments on conversion of BCP into FSC with the minimal sugar loss was assessed. Three different pretreatment strategies, namely, thermal, thermo-chemical, and MW-DA, with and without enzymatic saccharification, were evaluated (Fig. 1). The MW-DA pretreatment yielded the highest FSC (0.089 ± 0.004 g/g), whereas thermal and thermo-chemical pretreatments yielded FSC of 0.039 ± 0.000 and 0.077 ± 0.001 g/g, respectively (Fig. 1). These results indicate that different pretreatment methods had a partial effect on sugar release, but enzymatic saccharification was necessary to achieve optimal FSC. After enzymatic saccharification, FSC increased by 6.9 to 37% for thermal pretreatment and by 13.6 to 40% for thermo-chemical pretreatment. The combination of MW-DA treatment and enzymatic saccharification resulted in a significant enhancement of FSC yield, increasing it from 15.8 to 87.3%. Among all pretreatment strategies, MW-DA followed by enzymatic saccharification gave the maximum fermentable sugar production (0.493 g/g) with 87.3% yield (Fig. 1).
The influence of microwave power and pretreatment time on pretreatment and enzymatic saccharification conditions of BCP was examined using a Taguchi design. Initially, the FSC of untreated BCP was determined to be 0.565 ± 0.001 g/g biomass (Table 1). Subsequently, FSC from various pretreated samples without enzymatic saccharification ranged at 0.089 to 0.117 g/g. Remarkably, when BCP was subjected to MW-DA pretreatment followed by enzymatic saccharification, FSC values in the range of 0.339 to 0.515 g/g FSC were achieved. These FSC values were contingent on the specific pretreatment conditions, as detailed in Table 1. The highest FSC value (0.515 ± 0.002 g/g) was observed following MW-DA pretreatment at a MW power of 600 for 90 s, resulting in an enzymatic conversion rate of 22.69% (Table 1, MW6). These results align with findings from previous studies [26], which demonstrated that high FSC recovery can be achieved at relatively low MW powers (ranging from 100 to 150 W). However, it is noteworthy that an observed decrease in FSC recovery occurred when microwave power was increased to 600 and 850 W, with exceptions noted for MW-6 and 7 (Table 1).
In a related study, Binod et al. [27] investigated that fermentable sugar production using a microwave-assisted dilute acid strategy with sugarcane bagasse, reporting a fermentable sugar yield of 0.091 g/g under 1% sulfuric acid concentration at 600 W for 4 min. Similarly, Germec et al. [28] examined the effects of MW-DA conditions on fermentable sugar production from various biomass sources, including wheat bran, barley husk, oat husk, and rye bran, achieving a maximum fermentable sugar content of 49.65 g/L (equivalent to 0.83 g/g) from wheat bran at 700 W. Notably, the fermentable sugar content (0.493 g/g) achieved in our study following MW-DA pretreatment surpassed the results reported by Binod et al. [27].
Ethaib et al. [26] conducted research on three types of MW pretreatments followed by enzymatic saccharification of sago palm bark to investigate their effect on total reducing sugar. The maximum total fermentable sugar (0.386 g/g) was obtained after enzymatic saccharification at 5% solid/liquid ratio and 0.05 M H2SO4 under 440 W for 15 min. Zhu et al. [29] pretreated Miscanthus biomass and achieved a conversion rate of 75.3% under 0.2 M H2SO4 for 20 min at 300 W MW power, followed by saccharification using Celluclast and Novozyme 188. Remarkably, the fermentable sugar yield in our study (87.3%) after MW-DA pretreatment followed by enzymatic saccharification surpassed the results reported by both Zhu et al. [29] and Ethaib et al. [26].
Furthermore, it is crucial to consider the amount of energy consumed during pretreatment to achieve the maximum FSC. Table 1 provides a summary of energy consumption associated with each pretreatment, alongside the energy produced from the released sugars during hydrolysis stage. Notably, the lowest energy consumption (18.3 kJ/g) required to release 1 g of FSC was achieved using MW-DA at 300 W for 30 s. Conversely, the highest energy consumption (8612 kJ/g) was associated with thermal pretreatment at 121 °oC for 15 min. Interestingly, despite the application of higher energy levels to reach elevated temperatures in the autoclave, the FSC did not increase significantly with this pretreatment (Table 1). The FSC did not exhibit a linear or proportional increase with energy consumption at higher MW power levels (600 and 850 W) (Table 1).
Moreover, glucose, fructose, and arabinose were found as 0.750 ± 0.050 g/L, 0.004 ± 0.000 g/L, and 0.350 ± 0.020 g/L, respectively, in raw biomass. No xylose was detected in both raw material and pretreated sample. After MW-DA pretreatment and sequential enzymatic saccharification of BCP, glucose, fructose, and arabinose amounts were found as 2.33 ± 0.04 g/L, 1.50 ± 0.01 g/L, and 1.42 ± 0.11 g/L, respectively. Some inhibitors such as furfural, hydroxymethyl furfural (HMF), and acetic acid which inhibit the fermentation are formed during pretreatment of biomass [30]. At power, pretreatment time and acid concentration of 300 W, 30 s, and 2% (w/w) H2SO4, respectively, no HMF were detected in the hydrolyzate.
3.3 Evaluation of key variables affecting batch cultivation
3.3.1 Effect of different organic nitrogen sources on enzyme production
Nitrogen plays a vital role as a protein component for microbial growth and enzyme synthesis [31]. In this study, three nitrogen sources, namely, yeast extract, whey protein, and pea protein, were assessed to determine their impact on PG and PNL production. The results revealed varying effects on PG and PNL production, with yeast extract yielding 19.38 ± 0.32 U/L for PG and 22.00 ± 3.56 U/L for PNL, whey protein resulting in 14.67 ± 0.37 U/L for PG, and 8.55 ± 1.72 U/L for PNL, and pea protein demonstrating the most favorable outcomes with 20.50 ± 0.52 U/L for PG and 46.44 ± 3.45 U/L for PNL (Fig. 2). Among the tested organic nitrogen sources, pea protein proved to be the most suitable for both PG (20.5 ± 0.52 U/L) and PNL (46.44 ± 3.45 U/L) synthesis by B. subtilis (Fig. 2). It is worth noting that there was a linear increase in optical density (OD) with enzyme activity. Previous studies have also explored PG enzyme production by B. subtilis CM5 using various organic and inorganic nitrogen sources such as yeast extract, beef extract, peptone, urea, casein, ammonium chloride (NH4Cl), ammonium sulfate ((NH4)SO4), and ammonium acetate (CH3COONH4). The peptone exhibited the highest PG activity compared to other organic and inorganic nitrogen sources [32]. Similarly, Kaur and Gupta [11] reported that B. subtilis SAV 21 showed the highest pectinlyase activity when yeast extract was used among the other nitrogen sources such as peptone, beef extract, and ammonium salts. Oskay [33] focused on studying the production of polygalacturonase (PGase) by Aureobasidium pullulans P56 through submerged fermentation, utilizing agro-industrial residues like corn cob and wheat bran, alongside commercially available carbon and nitrogen sources such as yeast extract, peptone, tryptone, asparagine, alanine, potassium nitrate, ammonium sulphate, soybean meal, and urea. A PGase activity of 8.2 U/mL was achieved when using a combination of two nitrogen sources, namely, ammonium sulfate and yeast extract in equal proportions (1:1). However, when urea was employed as a nitrogen source, minimal enzyme production was observed.
3.3.2 Effect of incubation time on enzyme production
The investigation into the effect of incubation time on enzyme production revealed that the highest PG (139.33 ± 0.48 U/L) and PNL (140.96 ± 6.15 U/L) activity occurred after 60 h of fermentation at 35 °C, pH 7.0, and 120 rpm (Fig. 3). In a similar trend, Alqahtani et al. [34] reported that the maximum PG production using B. subtilis 15A-B92 was achieved at the 36th h under conditions of pH 4.5 and 40 °C. Esmail et al. [35] observed that the maximum PNL production from B. subtilis RSY7 was achieved at pH 8.5 and 40 °C after 72th h. Additionally, Murugan et al. [36] explored the potential of a newly isolated Bacillus species in terms of pectinase activity. Their findings revealed that Bacillus sp. FWS II-4 demonstrated optimal growth when incubated at pH 7.0 and 35 °C for 72 h.
It is worth noting that the optimal incubation time for pectinase production can vary depending on the specific microorganism used.
3.3.3 Effect of pea protein loading on enzyme production
The ratio of nitrogen to carbon (C/N ratio) is a critical factor in bioprocess media, influencing microbial growth and the production of value-added products [37, 38]. Excessive nitrogen supplementation can actually hinder enzyme production [39]. It is important to determine an optimal C/N ratio for maximum enzyme production [39]. In this study, it was found that the highest PG activity (109.43 ± 0.97 U/L) was achieved by adding 1.5 g of pea protein (represented as point C), while the highest PNL activity (101.85 ± 2.82 U/L) was observed with addition of 2 g of pea protein (point D) (Fig. 4). From an industrial perspective, the optimal nitrogen load for co-production of pectinase blend was selected as 1.5 g of pea protein. Abdollahzadeh et al. [40] explored the optimization of pectinase production by Enterobacter sp. MF84 using response surface methodology (RSM), with ammonium chloride (NH4Cl), dipotassium phosphate (K2HPO4), pectin, and incubation time as variables. Their findings indicated that as the concentration of ammonium chloride increased, particularly in low concentrations of pectin (5 g/L), there was a corresponding increase in enzyme production. These findings shed light on the significance of nitrogen source selection and nitrogen-carbon balance in enzyme production, offering insights that could be valuable in industrial applications.
3.4 Fed-batch cultivation with different BCP hydrolyzate feeding on enzyme production
Building upon the data obtained from the batch fermentation, a fed-batch fermentation was conducted in shake flasks. The cultivation commenced as a batch fermentation for the initial 54 h, during which cell growth, co-production of PG and PNL activity reached their maximum levels. Just before entering the stationary growth phase, sterile BCP hydrolyzate was introduced with varying feedings (2.5, 5, 10, and 15%). The pea protein concentration was maintained at a constant level of 1.5 g/100 mL.
The PG and PNL activity continued to increased and reached the maximal of 164.34 ± 2.26 U/L with 15% hydrolyzate feeding and 188.22 ± 1.72 U/L with 5% feeding after 72 h, respectively (Fig. 5). Interestingly, no statistically significant difference was observed between the 15 and 5% hydrolyzate feedings for PG activity (164.34 ± 2.26 U/L and 162.3 ± 2.28 U/L, respectively). Consequently, the hydrolyzate feeding rate was selected as 5% for both PG and PNL production in fed-batch fermentation.
Comparing different submerged cultivation strategies, such as batch and fed batch, in terms of PG and PNL production, it was observed that PG activity in the fed-batch cultivation system (164.34 ± 2.26 U/L) was higher than in batch cultivation system (139.33 ± 0.48 U/L) at pH 7.0. Furthermore, PNL activity was 140.96 ± 6.15 U/L at pH 7.0 using the batch system, while PNL activity reached 188.22 ± 1.72 U/L at the same pH using the fed-batch system (Fig. 5). Notably, PNL activity was found to be higher in the fed-batch system compared to the batch system (Fig. 5). Additionally, PNL activity surpassed PG activity. Thus, the fed-batch cultivation system exhibited greater efficiency than batch fermentation. Moreover, the maximum PG and PNL activities were observed at pH 7.0 in the fed-batch cultivation system with the addition of 5% substrate during the initial 6 h after fresh substrate loading. PG activity increased by 1.18-fold in the fed-batch fermentation, whereas PNL activity increased by 1.34-fold under the fed-batch fermentation conditions. Enhansy et al. [13] demonstrated that employing a bioreactor fed-batch cultivation method with continuous feeding of carbon source led to a significant 16.5-fold increase in maximal pectinase production compared to the initial conditions. Özısık [41] found that the highest result (12.27 U/mL) was achieved when the fermentation lasted for 3 days, with the addition of 1 g/L apple pomace as a carbon source on the second day of fermentation.
Upon introducing fresh BCP hydrolyzate (5%) into the fermentation media at the 54th h, bacterial growth increased, resulting in higher PG and PNL activities (Fig. 6). FSC and enzyme activities increased following the introduction of fresh substrate. These results demonstrated a significant enhancement in co-production of PG and PNL upon the addition of fermentable sugar. These findings align well with earlier studies in the literature. For instance, Zou et al. [6] reported that the maximum polygalacturonate lyase activity reached 19.6 U/mL after 38 h of fed-batch cultivation, compared to batch cultivation. Utilization of FSC occurred during fermentation, and a decrease in FSC was observed at the 60th h, coinciding with the increase in PG and PNL production proportionally (Fig. 6).
PG and PNL production, along with protein content, exhibited continuous increases until the 72th h, after which they began to decline (Fig. 6). Protein content is directly related to PG and PNL activity, as proteins are the building blocks of these enzymes. The highest total protein content (52.25 ± 0.06 mg/L) was observed at the 5% substrate loading level for both PG and PNL production. Zou et al. [6] noted that the enzyme activity and total protein content were directly proportional for polygalacturonate lyase from B. subtilis in the fed-batch system.
4 Conclusion
This study compared three different valorization strategies for BCP in terms of FSC, energy consumption, time efficiency, and formation of inhibitors. The highest FSC obtained was through MW-DA pretreatment, achieving 0.493 g/g with an 87.3% conversion yield. This result was achieved by applying 300 W for 30 s followed by an enzymatic saccharification step. MW-DA pretreatment, in conjunction with enzymatic saccharification, provided a substantial FSC yield in a short time while minimizing energy consumption and inhibitor formation.
Furthermore, this study investigated the medium composition for batch and fed-batch fermentation to produce pectinase blends using B. subtilis ATCC 6633, with carbon derived from BCP hydrolyzate and nitrogen sources, including pea protein. The results indicated that BCP hydrolyzate and pea protein had significant impacts on co-production of PG and PNL. Particularly, pea protein emerged as a promising nitrogen source for enhancing PG and PNL production. In batch fermentation, the highest activity achieved was 139.33 ± 0.48 U/L for PG and 140.96 ± 6.15 U/L for PNL activity. However, in the fed-batch fermentation, a maximal PG activity of 164.34 ± 2.26 U/L was attained at pH 7.0 with 5% hydrolyzate feeding, while the highest PNL activity reached 188.22 ± 1.72 U/L under the same culture conditions. This outcome suggests that the fed-batch method may be a more favorable option than the batch fermentation for co-producing pectinase enzyme preparations.
Data availability
Not applicable.
References
Pedrolli DB, Monteiro AC, Gomes E, Carmona EC (2009) Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes. The Open J 3:9–18
Wu M-C, Jiang C-M, Huang P-H, Wu M-Y, Wang YT (2007) Seperation and utilization of pectin lyase from commercial pectic enzyme via highly methoxylated cross-linked alcohol-insoluble solid chromatography for wine methanol reduction. J Agric Food Chem 55(4):1557–1562
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochem 40(9):2931–2944
Ravindran R, Jaiswal AK (2016) Microbial enzyme production using lignocellulosic food industry wastes as feedstock: a review. Bioeng 3:30
Costa AR, Rodrigues ME, Henriques M, Oliveira R, Azeredo J (2014) Feed optimization in fed-batch culture. Methods Mol Biol 1104:105–116
Zou M, Guo F, Li X, Zhao J, Qu Y (2014) Enhancing production of alkaline polygalacturonate lyase from bacillus subtilis by fed batch fermentation. Plos One 9:(3)
Kumar M, Dahuja A, Sachdev A, Kaur C, Varghese E, Saha S, Sairam KVSS (2019) Valorisation of black carrot pomace: microwave assisted extraction of bioactive phytoceuticals and antioxidant activity using Box–Behnken design. J Food Sci Technol 56:995–1007
Sabah MH, Al-Shatty AK, Niamah B, Abdullah Y (2011) The ability of Trichoderma harzianum on cleavage of cellulose of date palm leaves. J Tikrit Univ Agric Sci 11(4)
Agcam E, Akyildiz A (2015) Siyah Havuç Posasından Antosiyaninlerin Ekstraksiyonuna Farklı Çözgen ve Asit Konsantrasyonlarının Etkileri (Effects of different solvents and acid concentrations on extraction of anthocyanins from black carrot pomace). Food 40:149–156
Abdullah BY, Al-Kuzayi AKN, Al-Shatty SMH (2011) The ability of Trichoderma harzianum on cleavage of cellulose of date palm leaves. J Tikrit Univ Agric Sci 11(4)
Kaur SJ, Gupta VK (2017) Production of pectinolytic enzymes pectinase and pectin lyase by Bacillus subtilis SAV-21 in solid state fermentation. Annal Microbiol 67:333–342
Amande TJ, Ado BV, Adebayo-Tayo BC (2022) Production of fungal pectin lyase and polygalacturonase from fruit wastes by solid state fermentation. Biotech J Int 36(4):48–56
El Enshasy HA, Elsayed EA, Suhaimi N, Malek RA, Esawy M (2018) Bioprocess optimization for pectinase production using Aspergillus niger in a submerged cultivation system. BMC Biotech 18:71
Kapoor M, Kuhad RC (2002) Improved polygalacturonase production from Bacillus sp. MG-cp-2 under submerged (SmF) and solid state (SSF) fermentation. Lett Appl Microbiol 34(5):317–322
Ozzeybek M, Cekmecelioglu D (2022) Formulation of apple pomace, orange peel, and hazelnut shell mix for co-production of bacterial pectinase and cellulose enzymes by mixture design method. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02409-0
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. J Anal Chem 31:426–428
Lu X, Xi B, Zhang Y, Angelidaki I (2011) Microwave pretreatment of rape straw for bioethanol production: focus on energy efficiency. Bioresour Technol 102:7937–7940
AOAC (1984) Official methods of analysis. Association of Official Analytical Chemists, Washington, DC
Hara A, Radin NS (1978) Lipid extraction of tissues with a low toxicity solvent. Anal Biochem 90:420–426
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin Phenol Ragent. J Biol Chem 193:265–275
Sluiter A (2005) Determination of total solids in biomass. In: NREL Biomass Analysis Technology Team Laboratory Analytical Procedure. NREL, Golden
Xie R, Tu M, Wu Y, Adhikari S (2011) Improvement in HLPC separation of acetic acid and levulinic acid in the profiling of biomass hydrolysate. Bioresour Technol 102:4938–4942
Nedjma M, Hoffmann N, Belarbi A (2001) Selective and sensitive detection of pectin lyase activity using a colorimetric test: application to the screening of microorganism possessing pectin lyase activity. Anal Biochem 291:290–296
Elik A (2021) Hot-air assisted radio frequency drying of black carrot pomace: kinetics and product quality. Innov Food Sci Emerg Technol 73:102800
Ethaib S, Omar R, Mazlina MKS, Radiah ABD (2020) Evaluation of the interactive effect pretreatment parameters via three types of microwave-assisted pretreatment and enzymatic hydrolysis on sugar yield. Processes 8:787
Binod P, Karri S, Raveendran S (2012) Short duration microwave assisted pretreatment enhances the enzymatic sachharification and fermentable sugar yield from sugarcane bagasse. Renew Energy 37(37):109–116
Germec M, Demirel F, Tas N, Ozcan A, Yılmazer C, Onuk Z, Turhan İ (2017) Microwave-assisted dilute acid pretreatment of different agricultural bioresources for fermentable sugar production. Cellulose 24:4337–4353
Zhu Z, Macquarrie DJ, Simister R, Gomez LD, Mc-Queen-Mason SJ (2015) Microwave assisted chemical pretreatment of Miscanthus under different temperature regimes. Sustain Chem Process 3(15):2–13
Karunanithy C, Muthukumarappan K (2011) Optimization of alkali soaking and extrusion pretreatment of prairie cord grass for maximum sugar recovery by enzymatic hydrolysis. Biochem Eng J 54:71–82
Atala ML, Ali LF, Kadhim MJ (2015) Optimization of pectinase production from Pseudomonas sp. isolated from Iraqi soil. Iraqi J Sci 56(3):2595–2600
Swain MR, Ray RC (2010) Production, Characterization and application of a thermostable exo-polygalacturonase by Bacillus subtilis CM5. Food Biotechnol 24(1):37–50
Oskay M (2022) Production, partial purification, and characterization of polygalacturonase from Aureobasidium pullulans P56 under submerged fermentation using agro-industrial wastes. Current Microbiol 79:296
Alqahtani YS, More SS, Keerthana R, Shaikh IA, Anusha KJ, More VS, Niyonzima N, Muddapur UM, Khan AA (2022) Production and purification of pectinase from Bacillus subtilis 15a-b92 and its biotechnological applications. Molecules 27(13):4195
Esmail R, Yazji S, Balaa BA (2013) Isolation, production and characterization of extracellular pectin lyase from Bacillus subtilis. Adv Environ Biol 7(13):3917–3924
Murugan T, Deepika P, Kowsalya A, Sivasubramanian K, Rejisha RP, Murugan M, Wins JA (2021) Production and characterization of extracellular pectinase from a newly isolated Bacillus species from fruit waste soil. Mater Today:Proc 45: 2087-2090
Ulhiza TA, Puad NIM, Azmi AS (2018) Optimization of culture conditions for biohydrogen production from sago wastewater by Enterobacter aerogenes using response surface methodology. Int J Hydrog Energy 43:22148–22158
Xiao R, Li X, Zheng Y (2018) Enzyme production by a fungoid marine protest Thraustochytrium striatum. Eur J Protistol 66:136–148
Panesar PS, Chavan Y, Chopra HK, Kennedy JF (2012) Production of microbial cellulose: response surface methodology approach. Carbohydr Polym 87:930–934
Abdollahzadeh R, Pazhang M, Najavand S, Fallahzadeh-Mamaghani V, Amani_Ghadim AR (2020) Screening of pectinase-producing bacteria from farmlands and optimization of enzyme production from selected strain by RSM. Folia Microbiol 65: 705-719
Ozısık B (2023) Bacterial polygalacturonase production using apple pomace bu submerged fermentation. MSc Thesis in Biotechnology. METU, Ankara, Turkey, pp 1–99
Acknowledgements
The authors would like to thank Dr. Tuğba Doğan and Sevil Erkut at the Middle East Technical University Central Laboratory, Ankara, Turkey, for assistance with HPLC analyses and Dane Ruscuklu at the Izmir Instıtute of Technology the Center of Biotechnology and Bioengineering Application and Research (IZTECH-CFB), Izmir, Turkey.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). Funding for this study was provided by IZTECH Research Fund Project, 2022IYTE-1-0005, at the Department of Food Engineering, Izmir Instıtute of Technology, Turkey.
Author information
Authors and Affiliations
Contributions
Busra Nur Esen: performed the experiments or data/evidence collection; Sibel Uzuner: conducted a research and investigation process, specifically performed the experiments, or data/evidence collection, formal analysis (application of statistical or mathematical techniques to analyze or synthesize study data), and wrote—original draft (preparation and/or creation of the published work, specifically writing the initial draft); Hümeyra Taşkent Sezgin: wrote—review and edited specifically critical review, commentary, or revision, including pre- or post-publication stages.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esen, B.N., Uzuner, S. & Sezgin, H.T. Valorization of black carrot pomace and pea powder for co-production of polygalacturonase and pectin lyase. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05646-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05646-7