Abstract
This research aims to explore the potential use of carbon derived from neem bark (NBC) for the effective removal of Congo red (CR) dye from water-based solutions. A series of batch adsorption experiments were conducted to examine the impact of various factors. Subsequently, the optimal conditions were identified. The determination of the ideal operational conditions for employing NBC involved several important factors, namely agitation time: 60 min, dosage: 2 g/L, temperature: 45° C, and CR concentration: 40 mg/L. A comprehensive investigation was conducted to enhance the understanding of the composition and physical properties of the adsorbent NBC using Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), and Brunauer–Emmett–Teller (BET) analysis. The FTIR analysis detected notable changes in the spectral peaks pre- and post-adsorption. The BET analysis provided significant results regarding the surface area, total pore volume, and average pore diameter of the adsorbents. The Langmuir isotherm model was found to be more accurate in fitting the experimental data with adsorption capacity obtained as 25.84 mg/g. The kinetic investigations yielded results that conformed to the pseudo-second-order model with rate constant 1.03009 \(\times\) 10−3 g/mg min. The thermodynamic evaluations in this study indicate that the adsorption phenomena are exothermic, thermodynamically favourable, and spontaneous. In conclusion, this research demonstrates the effectiveness of NBC as an adsorbent for removing CR dye from textile industry effluent. The findings offer new perspectives on addressing water pollution and highlight the feasibility of using sustainable adsorbents for environmental remediation.
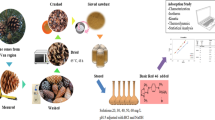
Graphical Abstract









Similar content being viewed by others
Data availability
All the data and materials are presented within the manuscript.
References
Vinayagam R, Kar A, Murugesan G, Varadavenkatesan T, Goveas LC, Samanth A, Ahmed MB, Selvaraj R (2023) Low temperature carbonized mesoporous graphitic carbon for tetracycline adsorption: mechanistic insight and adaptive neuro-fuzzy inference system modeling. Bioresour Technol Reports 22:101468. https://doi.org/10.1016/j.biteb.2023.101468
Samanth A, Vinayagam R, Murugesan G, Varadavenkatesan T, Selvaraj R, Pugazhendhi A (2023) Enhanced adsorption of 2,4-dichlorophenoxyacetic acid using low-temperature carbonized Peltophorum pterocarpum pods and its statistical physics modeling. Chemosphere 336:139143. https://doi.org/10.1016/j.chemosphere.2023.139143
Ali NS, Majdi HS, Albayati TM, Jasim DJ (2023) Adsorption of aniline from aqueous solutions onto a nanoporous material adsorbent: isotherms, kinetics, and mass transfer mechanisms. Water Pract Technol 18:2136–2150. https://doi.org/10.2166/wpt.2023.132
Hnamte M, Pulikkal AK (2022) Clay-polymer nanocomposites for water and wastewater treatment: a comprehensive review. Chemosphere 307:135869. https://doi.org/10.1016/j.chemosphere.2022.135869
Ganea I-V, Nan A, Baciu C, Turcu R (2021) Effective removal of crystal violet dye using neoteric magnetic nanostructures based on functionalized poly (benzofuran-co-arylacetic acid): investigation of the adsorption behaviour and reusability. Nanomaterials 11(3):679. https://doi.org/10.3390/nano11030679
Al-Jaaf HJ, Ali NS, Alardhi SM (2022) Albayati TM Implementing eggplant peels as an efficient bio-adsorbent for treatment of oily domestic wastewater. Desalin Water Treat 245:226–237. https://doi.org/10.5004/dwt.2022.27986
Saniya A, Sathya K, Nagarajan K, Jayalakshmi H, Bharathi S (2020) Optimization of synthesis of biochar for the removal of cationic dye from aqueous solution. Int J Adv Res 8(2):84–89. https://doi.org/10.21474/IJAR01/10438
Saniya A, Sathya K, Nagarajan K, Yogesh M, Jayalakshmi H, Praveena P, Bharathi S (2020) Modelling of the removal of crystal violet dye from textile effluent using Murrayakoenigii stem biochar. Desalin Water Treat 203:356–365. https://doi.org/10.5004/dwt.2020.26191
Sharada S, Bhagya Sri N, Supriya K, Venkatesh M, Srihari M (2016) Adsorption of toxicants (chromium, iron and oxalic acid) on activated carbons prepared from tamarind seeds. Int J Sci Dev Res 1(5):274–283
Jamion NA, Hashim IN (2017) Preparation of activated carbon from tamarind seeds and methylene blue (MB) removal. J Fundam Appl Sci 9(6S):102–114. https://doi.org/10.4314/jfas.v9i6s.9
Sridevi H, Ramananda Bhat M, Selvaraj R (2023) Removal of an agricultural herbicide (2,4-Dichlorophenoxyacetic acid) using magnetic nanocomposite: a combined experimental and modeling studies. Environ Res 238:117124. https://doi.org/10.1016/j.envres.2023.117124
Zheng Y, Cheng B, Fan J, Jiaguo Yu, Ho W (2021) Review on nickel-based adsorption materials for congo red. J Hazard Mater 403:123559. https://doi.org/10.1016/j.jhazmat.2020.123559
AjmalKoyaPulikkal NL, Anjudikkal J (2023) Effective adsorption of polycyclic aromatic Congo red dye by modified garlic peel. J Dispers Sci Technol 0:1–11. https://doi.org/10.1080/01932691.2023.2181180
Hussain MS, Rehman R, Imran M, Dar A, Akram M, Al-Abbad EA (2022) Eco-friendly detoxification of congo red dye from water by citric acid activated bioadsorbents consisting of watermelon and water chestnuts peels collected from indigenous resources. Adsorption Sci Technol 2022(9056288):20. https://doi.org/10.1155/2022/9056288
Dabagh A, Benhiti R, Abali M, Ichou AA, Sinan F, Zerbet M (2023) Valorization of plant biomass by chemical pre-treatment: application to the removal of Rhodamine B and Congo Red dyes. Biomass Conv Bioref. https://doi.org/10.1007/s13399-023-04299-2
Alamrani NA, AL-Aoh HA (2021) Elimination of Congo Red Dye from industrial wastewater using Teucrium polium L. as a low-cost local adsorbent. Adsorption Sci Technol 2021(5728696):12. https://doi.org/10.1155/2021/5728696
Ravikumar M, King P (2020) Application of response surface optimization on biosorption of Congo red dye onto Spathodeacampanulata leaves. Desalin Water Treat 182:342–350. https://doi.org/10.5004/dwt.2020.25140
Wekoye JN, Wanyonyi WC, Wangila PT, Tonui MK (2020) Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environ Chem Ecotoxicol 2:24–31. https://doi.org/10.1016/j.enceco.2020.01.004
Khamis Soliman N, Moustafa AF, Aboud AA, Halim KSA (2019) Effective utilization of Moringa seeds waste as a new green environmental adsorbent for removal of industrial toxic dyes. J Mater Res Technol 8(2):1798–1808
Vairavel P, Rampal N, Jeppu G (2021) Adsorption of toxic Congo red dye from aqueous solution using untreated coffee husks: kinetics, equilibrium, thermodynamics and desorption study, Int J Environ Anal Chem 1–20 https://doi.org/10.1080/03067319.2021.1897982
Litefti K, Freire M, Stitou M, Alvarez JG (2019) Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci Rep 9:16530. https://doi.org/10.1038/s41598-019-53046-z
Lafi R, Montasser I (2019) Hafiane A Adsorption of Congo red dye from aqueous solutions by prepared activated carbon with oxygen-containing functional groups and its regeneration. Adsorpt Sci Technol 37(1–2):160–181
Virk AK, Thakur P, Sharma I (2020) Swati A Moringa oleifera seeds-based filter for efficient removal of Congo red from aqueous medium. Des Water Treat 206:371–384. https://doi.org/10.5004/dwt.2020.26251
Dandge R, Ubale M, Farooqui M, Rathod S (2016) Adsorption study for the removal of hazardous dye Congo red by biowaste materials as adsorbents. Int J Appl Innov Eng Manage 5(11):9–16
Senthilkumar R, Reddy Prasad DM, Govindarajan L, Saravanakumar K, Naveen Prasad BS (2020) Synthesis of green marine algal-based biochar for remediation of arsenic(V) from contaminated waters in batch and column mode of operation. Int J Phytorem 22(3):279–286
Saravanakumar K, Senthilkumar R, Prasad DMR, Prasad BSN, Manickam S, Gajendiran V (2020) Batch and column arsenate sorption using turbinaria ornata seaweed derived biochar: experimental studies and mathematical modeling. ChemistrySelect 5(12):3661–3668
Sowjanya B, King P, Vangalapati M, Myneni VR (2023) Copper-doped zinc oxide nanoparticles: synthesis, characterization, and application for adsorptive removal of toxic azo dye. Int J Chem Eng 2023(8640288):13. https://doi.org/10.1155/2023/8640288
Senthilkumar K, Neeraja M, Prathap S, Vasant M, Naveenkumar M, Venkata Ratnam M (2023) Adsorption of methylene blue dye by animal dung biomass–derived activated carbon: optimization, isotherms and kinetic studies. Biomass Conv Bioref. https://doi.org/10.1007/s13399-023-04710-y
Myneni VR, Kanidarapu NR, Shaik F, Meena V (2022) Response surface modeling of the Removal of methyl orange dye from aqueous solution using magnesium oxide nanoparticles immobilized on chitosan. Iran J Chem Chem Eng 41(5):1602–1618. https://doi.org/10.30492/ijcce.2021.122511.4002
Prabha PL, Rani SAF, Jayalakshmi B, Ramachandramoorthy T (2016) Curry tree carbon—a novel adsorbent for the removal of Zn(II) ions in aqueous medium. World J Pharm Pharm Sci 5(3):1543–1557
Azeez RA, Al-Zuhairi FKI (2022) Biosorption of dye by immobilized yeast cells on the surface of magnetic nanoparticles. Alex En J 61(7):5213–5222. https://doi.org/10.1016/j.aej.2021.10.044
Mahdi AE, Ali NS, Majdi HS et al (2023) Effective adsorption of 2-nitroaniline from wastewater applying mesoporous material MCM-48: equilibrium, isotherm, and mechanism investigation. Desalin Water Treat 300:120–129. https://doi.org/10.5004/dwt.2023.29741
Ozturk A, Bayol E, Abdullah MI (2020) Characterization of the biosorption of fast black azo dye K salt by the bacterium Rhodopseudomonas palustris 51ATA strain. Electron J Biotechnol 46:22–29. https://doi.org/10.1016/j.ejbt.2020.05.002
Abbas M (2020) Removal of brilliant green (BG) by activated carbon derived from medlar nucleus (ACMN)—kinetic, isotherms and thermodynamic aspects of adsorption. Adsorpt Sci Technol 38(9–10):464–482. https://doi.org/10.1177/0263617420957829
Ibisi NE, Asoluka CA (2018) Use of agro-waste (Musa paradisiaca peels) as a sustainable biosorbent for toxic metal ions removal from contaminated water. Chem Int 4:52–59. https://doi.org/10.5281/zenodo.1475334
Gogoi AJ, Pulikkal AK (2022) Clay–gemini surfactant hybrid materials for elimination of inorganic pollutants: a comprehensive review. Results Chem 4:100586. https://doi.org/10.1016/j.rechem.2022.100586
Lahreche S, Imane M, El Kebir A, Sabantina L, Kaid M, Benyoucef A (2022) Application of activated carbon adsorbents prepared from prickly pear fruit seeds and a conductive polymer matrix to remove Congo red from aqueous solutions. Fibers 10(1):7. https://doi.org/10.3390/fib10010007
Abbood NS, Ali NS, Khader EH et al (2023) Photocatalytic degradation of cefotaxime pharmaceutical compounds onto a modified nanocatalyst. Res Chem Intermed 49:43–56. https://doi.org/10.1007/s11164-022-04879-3
Ahmed Aftab R, Zaidi S, Aslam Parwaz Khan A et al (2023) Removal of congo red from water by adsorption onto activated carbon derived from waste black cardamom peels and machine learning modeling. Alexandria Eng J 71:355–369. https://doi.org/10.1016/j.aej.2023.03.055
Acknowledgements
The authors thank the institute authorities for providing the facilities to carry out the project work.
Author information
Authors and Affiliations
Contributions
KS—conceptualization, methodology, validation, formal analysis, supervision, writing—review and editing.
HJ—conceptualization, methodology, writing—original draft.
SNR—conceptualization, validation, supervision.
MVR—software, writing—original draft, writing—review and editing.
DB—writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
The study does not include human/animal objects. Hence, no approvals are needed.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sathya, K., Jayalakshmi, H., Reddy, S.N. et al. Effective removal of Congo red dye using adsorbent prepared from bio-waste: isotherm, kinetic, and thermodynamic studies. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05213-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05213-6