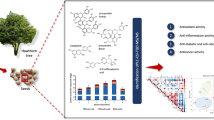
Abstract
This study was undertaken to explore the content and the chemical constituents of the essential oils (EOs) and methanol leaf extracts (MLEs) from Corymbia citriodora, Cupressus macrocarpa, and Syzygium cumini. Chromatographic analyses of GC–MS and HPLC were used. The EOs and MLEs were prepared at concentrations of 0, 6, 12, 25, and 50 mg/L, while the extract was prepared at concentrations of 0, 500, 1000, 2000, and 4000 mg/L. Sapwood blocks of Pinus sylvestris in the dimension of 0.5 × 2 × 2 cm were prepared and autoclaved at 121 °C, and each wood block received 100 µL of the prepared concentrations from the EOs and MEs. The bioactivities of wood-treated EOs or MEs were measured against the growth of Fusarium solani MW947256. By GC–MS, the main compounds in the EOs from C. citriodora were citronellal, citronellol, p-cymene, spathulenol, and isopulegol with values of 23.95, 9.80, 9.32, 9.29, and 5.38%, respectively, in Cup. macrocarpa leaves were sabinene (11.94%), 4-terpinenol (11.34%), citronellol (9.59%), citronellal (9.85%), p-cymene (7.67%), spathulenol (5.24%), γ-terpinene (5.05%), camphor (4.31%), and limonene (3.2%), and in S. cumini leaves were trans-β-ocimene (19.11%), α-pinene (18.79%), caryophyllene (9.30%), (Z)-β-ocimene (8.16%), and limonene (6%). By HPLC, the most abundant phenolic compounds in the methanol extract from C. citriodora benzoic acid (8.11 μg/g), and gallic acid (7.96 μg/g), from Cup. macrocarpa were syringic acid (7.59 μg/g), catechol (6.85 μg/g), and gallic acid (6.78 μg/g), and from S. cumini were cinnamic acid (10.66 μg/g), caffeic acid (9.87 μg/g), and ellagic acid (8.76 μg/g). The highest percentages of inhibition (65.71% and 35.71%) against the growth of F. solani were seen in the wood treated with Cup. macrocarpa EOs at 50 and 25 mg/L, respectively. The maximum level of inhibition was seen (92.85%) when S. cumini MLEs at a dose of 4000 mg/L was applied to wood samples, followed by Cup. macrocarpa MLEs (70.00%) compared to the positive control of azoxystrobin + difenoconazole (1000 mg/L), which caused 100% inhibition to F. solani. The findings indicated that bioactive chemicals present in the extracts and EOs from these trees have strong antifungal properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plant essential oils (EOs) and extracts play a potential role as alternatives to fungicidal agents that are currently used because of their contact inhibition, penetration, and volatile effect. Several EOs have the potential to control different fungal species and can exert strong antifungal activities [1,2,3,4,5].
Corymbia citriodora (Hook.) K.D.Hill & L.A.S.Johnson or Eucalyptus citriodora Hook., the lemon-scented gum, its EOs, and phenolic and flavonoid compounds have great potential uses as antimicrobial and antioxidant activities [6,7,8,9]. A qualitative phytochemical analysis was performed for the detection of alkaloids, cardiac glycosides, flavonoids, saponins, sterols, tannins, and phenols [10]. Phytochemical studies on E. citriodora kino have described the isolation of triterpenoids, phenolics, and flavonoids [11, 12]. Leaf extract showed promising values against Staphylococcus aureus [13]. Citronellal, citronellyl acetate, and trans-caryophyllene were the major compounds in the leaf EO and showed the highest antibacterial activity towards Staphylococcus aureus and Bacillus subtilis and antifungal activity against Aspergillus fumigatus and Saccharomyces cerevisiae [14]. C. citriodora possesses a slight cytotoxic effect which is observed in their study. Leaf extract of plants can delay the loss of climbing ability in drosophila flies [15].
Cupressus macrocarpa Hartweg. ex Gordon (Callitropsis macrocarpa) (family Cupressaceae), or Monterey Cypress [16, 17], is traditionally used in rheumatism, whooping cough, styptic problem, improve bladder tone, and coadjuvant [18, 19]. Various phytoconstituents revealed the presence of carbohydrates, anthraquinone glycosides, cardiac glycosides, phenolics, EOs, flavonoids, saponin, protein, amino acid, and sterols in different extracts [20]. This plant contains diterpenes, monoterpenes, biflavonoids, and sesquiterpenes [9, 21,22,23]. The biflavonoid isolated from Cup. macrocarpa was shown a hepato- and nephro-protective activity in mice against carbon tetrachlo-ride-induced toxicity [23].
Syzygium cumini (L.) Skeels (jambolan) or Eugenia jambolana, common plum and java plum, is one of the widely used medicinal plants [24]. The leaf EO of S. cumini was found to be good antibacterial activity [25]. The EO showed significant activity against Leishmania amazonensis [26]. Leaf EO from S. cumini with its main α-caryophyllene, β-caryophyllene, and terpineol showed antimycobacterial activity [27].
Extracts and EOs from several medicinal and higher plants have been used to protect wood and other wood-based products and objects from mold infestations [28]. In this context, the inhibitory effects of eighteen EOs isolated from Egyptian plants were evaluated against two wood decay fungi, Ganoderma lucidum and Hexagonia apiaria. The EOs revealed various mycelial growth inhibitions against the tested fungi depending on fungal species and tested EOs [29]. The use of natural substances that do not have undesirable side effects in place of harmful ways and the implementation of safe, unique substances have been the main topics of new and alternative research [30]. Many plants have natural chemical components such as EOs, phenols, flavonoids, alkaloids, coumarins, and tannins that are employed as nutrition, medicines, and bioactive molecules [31]. Plant-derived natural products may be the ideal solution for safely halting the biodegradation of wood [32,33,34,35].
In order to produce a useful practical approach for preventing the growth of fungi on wood, this work seeks to compile the use of pure essential oils and extracts from the trees Corymbia citriodora, Cupressus macrocarpa, and Syzygium cumini. This was accomplished by applying extracts and essential oils to Scots pine sapwood (Pinus sylvestris) and testing them against the growth of the fungus Fusarium solani. For additional analytical techniques, GC–MS and HPLC were employed to identify chemical components.
2 Materials and methods
2.1 Plant material
Intact fresh leaves of three trees namely Corymbia citriodora, Cupressus macrocarpa, and Syzygium cumini were collected from Alexandria, Egypt, where C. macrocarpa and S. cumini were collected from the garden of the Faculty of Agriculture, Alexandria University, and the leaves from C. citriodora were collected from a plantation located at Alexandria-Cairo desert road (Albostan area), Alexandria, Egypt. The collected trees have been identified at the Department of Forestry and Wood Technology, Faculty of Agriculture, Alexandria University, Alexandria, Egypt, with their voucher numbers Z007, Z008, and Z009, for C. citriodora, Cup. macrocarpa, and S. cumini, respectively.
2.2 Extraction of the essential oil
The extraction process of the essential oils (EOs) from the fresh leaves of three trees C. citriodora, Cup. macrocarpa, and S. cumini. Plant material of about 100 g was washed using tap water and then cut into small pieces using scissors and put into a 2-L flask containing 1000 mL of distilled water (DW) and extracted by the hydrodistillation method using a Clevenger-type apparatus for 3 h [36]. After obtaining the EOs, they were over anhydrous sodium sulfate and kept dry in sealed Eppendorf tubes stored at 4 °C until chemical and bioassay analyses. The yields of EOs were 4.5, 2.5, and 0.08 mL/100 g plant fresh leaves from C. citriodora, Cup. macrocarpa, and S. cumini, respectively.
2.3 Gas chromatography–mass spectrometry (GC–MS) analysis of the essential oil
The chemical composition of the EOs was performed using Trace GC-TSQ mass spectrometer (Thermo Scientific, Austin, TX, USA) with a direct capillary column TG–5MS (30 m × 0.25 mm × 0.25 µm film thickness). The column oven temperature was initially held at 50 °C and then increased by 5 °C/min to 250 °C held for 2 min and increased to the final temperature of 300 °C by 30 °C/min and held for 2 min. The injector and MS transfer line temperatures were kept at 270 and 260 °C, respectively. Helium was used as a carrier gas at a constant flow rate of 1 ml/min. The solvent delay was 2 min and diluted samples of 1 µL were injected automatically using Autosampler AS1300 coupled with GC in the split mode. EI mass spectra were collected at 70 eV ionization voltages over the range of m/z 50–650 in full scan mode. The ion source temperature was set at 200 °C. The components were identified by comparison of their mass spectra with those of WILEY 09 and NIST 14 mass spectral database [37]. Xcalibur 3.0 data system in the GC–MS with its threshold values was used to confirm that all the mass spectra of the identified compounds were attached to the library. Furthermore, the measurement match factor (MF) with values ≥ 650 was used to confirm the identified compounds [38].
All analyses of studied samples have been done using Thermo Fisher GC–MS, which is supported by a number of mass spectral libraries, and all separated compounds have been identified and all data have been reported using NIST014, which is already supported by retention index (RI) library for 82,868 compounds (https://www.nist.gov/system/files/documents/srd/NIST1aVer22Man.pdf) [39, 40].
2.4 Methanol extracts
Leaves from the three tree species (C. citriodora, Cup. macrocarpa, and S. cumini) were air-dried at room temperature for 1 week, thin cut to small sizes or particles using a small laboratory mill. Approximately 50 g of each leaf sample was soaked in about 100 mL of methanol solvent in a conical flask for 1 week and then filtered through a cotton plug and Whatman no. 1 filter paper. The solvent evaporated as the extract was poured into Petri dishes to complete the dryness and then concentrated [41]; then, the methanol leaf extracts (MLEs) were obtained. The yields of the methanol extracts were 8.2, 6.6, and 9.12 g/100 g air-dry leaves from C. citriodora, Cup. macrocarpa, and S. cumini, respectively.
2.5 HPLC analysis of phenolic compounds
For the phytochemical analysis, the phenolic compounds from the MEs of C. citriodora, Cup. macrocarpa, and S. cumini leaves were identified by HPLC (Agilent 1100). The instrument was composed of a binary LC pump, a UV/Vis detector, and a C18 column (125 mm × 4.60 mm, 5-µm particle size) [42]. The Agilent Chem-Station was used to acquire and analyze chromatograms. A gradient mobile phase of two solvents—Solvent A (MeOH) and Solvent B [acetic acid in H2O (1:25)]—was used to separate phenolic acids. The gradient program began with 100% B and remained there for 3 min. This was followed by 5 min of 50% eluent A, 2 min of 80% eluent A, 5 min of 50% eluent A, 2 min of 80% eluent A, 5 min of 50% eluent A, 5 min of 50% eluent A, and 5 min of detection wavelength at 250 nm. As a result, the phenolic components were arranged in order to authenticate standard components by this mobile phase.
2.6 Antifungal activity of wood-treated essential oils and leaf methanol extracts
Air-dried Scots pine (Pinus sylvestris L.) sapwood was used for the treatment with the EOs and the MEs. The identification of wood was performed by academicals staff in the Forestry and Wood Technology Department, Faculty of Agriculture, Alexandria University using softwood key identification [43]. Microscopic cross section was examined (Fig. 1).
The EOs were prepared at the concentrations of 0, 6, 12, 25, and 50 mg/L, while the MEs were prepared at 0, 500, 1000, and 4000 mg/L by dissolving the respective amount in dimethylsulfoxide (DMSO 10%), and a few drops of Tween 80 was added for the concentrated EOs. Air-dried Scots pine sapwood blocks with dimensions of 0.5 cm × 2 cm × 2 cm were autoclaved at 121 °C for 20 min, then treated with the prepared concentrations of EOs and MEs (each block has received a 100 μL). The fungus Fusarium solani MW947256 was assayed for linear growths as incubated with the treated woods in the incubator chamber at 20–25 °C with relative humidity 85–92% for 7 days; then, the percentage of mycelial growth inhibition was measured with the following formula [44]: MGI = [(Ac − At) / Ac] × 100; where MGI is mycelial growth inhibition and AC and AT are average diameters of the fungal colony of the control and treatment, respectively. Wood samples soaked only with 10% DMSO were used as the negative control. Positive control of azoxystrobin + difenoconazole (1000 mg/L) was also employed.
2.7 Statistical analysis
The obtained data from the antifungal activity of essential oils and methanol extracts from the leaves of the three trees with their concentrations were analyzed using CoStat software version 6.303 [45], in split-plot design. The factor concentrations were set as the main plot, while the factor EOs or MEs as well as the interactions between the source and concentrations of EOs or MEs. The least significant difference at 0.05 level of probability was used for comparison between the means [46].
3 Results
3.1 Chemical compositions of the essential oils
Table 1 and Fig. 2 present the chemical compounds identified in the essential oil (EO) from Corymbia citriodora. The main compounds were citronellal (23.95%), citronellol (9.80%), p-cymene (9.32%), spathulenol (9.29%), isopulegol (5.38%), crypton (4.65%), citronellol epoxide (4.45%), neoisopulegol (3.67%), citronellic acid (2.96%), eucalyptol (2.7%), 4-terpinenol (2.36%), citronellol acetate (2%), trans-3-nonen-2-one (1.48%), and cuminaldehyde (1.21%).
The chemical constituents of the EO from Cupressus macrocarpa leaves are shown in Table 2. The main abundant compounds were sabinene (11.94%), 4-terpinenol (11.34%), citronellol (9.59%), citronellal (9.85%), p-cymene (7.67%), spathulenol (5.24%), γ-terpinene (5.05%), camphor (4.31%), limonene (3.2%), α-terpinene (2.89%), β-mmyrcene (2.64%), 15-kaurene (2.4%), terpinolene (2.32%), sclareol (1.86%), linalool (1.61%), α-terpineol (1.53%), farnesol (1.48%), crypton (1.32%) and α-thujene (1.03%).
Table 3 shows the chemical compounds of the EO from Syzygium cumini leaves. The main compounds were trans-β-ocimene (19.11%), α-pinene (18.79%), caryophyllene (9.30%), (Z)-β-ocimene (8.16%), limonene (6.00%), humulene (4.69%), β-pinene (4.01%), α-terpineol (3.79%), bornyl acetate (2.88%), myrcene (2.26%), citronellal (1.79%), 2,2,4a,7a-tetramethyldecahydro-1H-cyclobuta[e]inden-5-ol (1.76%), caryophyllenyl alcohol (1.63%), globulol (1.62%), terpinen-4-ol (1.62%), caryophyllene oxide (1.54%), and citronellol (1.33%).
3.2 Phenolic compounds
Results from Table 4 and Fig. 3 show the identified phenolic compounds in the methanol leaf extracts (MLEs) of C. citriodora (Fig. 2a), Cup. macrocarpa (Fig. 2b), and S. cumini (Fig. 2c). Benzoic acid (8.11 μg/g), gallic acid (7.96 μg/g), ellagic acid (5.78 μg/g), caffeic acid (4.65 μg/g), and catechol (3.75 μg/g) were the most abundant compounds in the MLE of C. citriodora. The abundant phenolic compounds in the MLE from Cup. macrocarpa were syringic acid (7.59 μg/g), catechol (6.85 μg/g), gallic acid (6.78 μg/g), and chlorogenic acid (4.62 μg/g). In the MLE of S. cumini, the most abundant compounds were cinnamic acid (10.66 μg/g) caffeic acid (9.87 μg/g), and ellagic acid (8.76 μg/g).
3.3 Antifungal activity of essential oils and methanol extracts against Fusarium solani
The antifungal activity of Scots pine treated with EOs and MLE against the growth of Fusarium solani is visually observed in Figs. 4, 5, and 6. The growth of the fungus mycelium was inhibited as EO or MLE concentrations were increased, compared to the positive control of azoxystrobin + difenoconazole (1000 mg/L), which caused 100% inhibition to F. solani.
Figure 7 illustrates the main impacts of EO concentration and source on F. solani development. The percentage of fungal mycelial growth inhibition increased as the concentrations rose. Additionally, Cup. macrocarpa EO was shown to have the highest overall impacts of any EO source. Table 5 shows the proportion of F. solani mycelial growth that was inhibited (%) when wood was treated with different EO doses. The wood-treated Cup. macrocarpa EO at 50 and 25 mg/L, respectively, showed the highest levels of inhibition (65.71% and 35.71%) against the growth of F. solani. Additionally, S. cumini EO and C. citriodora EO both inhibited F. solani at 50 mg/L (35.71% and 35.23%, respectively). Additionally, moderate activity was found when the EO from Cup. macrocarpa EO was applied at 12 mg/L with an inhibition value of 28.57%, and from C. citriodora EO with a value of 22.85% at 25 mg/L.
The main impacts of MLE concentration and their sources on F. solani growth were displayed in Fig. 8. It is evident that fungal inhibition (%) increased as MLE concentration rose. Furthermore, out of all the examined extracts, S. cumini MLE displayed the highest overall inhibition. Table 6 displays how MLEs applied to wood inhibited the fungal mycelium growth of F. solani. It is interesting to note that S. cumini MLE at a concentration of 4000 mg/L when applied to wood samples demonstrated the highest level of inhibition (92.85%), followed by Cup. macrocarpa MLE (70%). The wood samples treated with C. citriodora MLE at 4000 mg/L (49.52%), 2000 mg/L (41.90%), Cup. macrocarpa MLE (37.14%), and S. cumini MLE (35.71%) at 2000 mg/L showed good efficacy against the growth of F. solani as well.
4 Discussion
The EOs and phenolic compound profiles from the leaves of three Egyptian-grown trees Corymbia citriodora, Cupressus macrocarpa, and Syzygium cumini were examined in the current study using chromatographic equipment for GC–MS and HPLC. When applied to wood samples, the bioactivities against the growth of Fusarium oxysporum were likewise documented with varying degrees of inhibition.
C. citriodora EO demonstrated the existence of citronellal, citronellol, p-cymene, spathulenol, isopulegol, crypton, citronellol epoxide, neoisopulegol, citronellic acid, eucalyptol, 4-terpinenol, citronellol acetate, trans-3-nonen-2-one, and cuminaldehyde as major components. Citronellal (78%) was the main component for C. citriodora EO grown in Portugal [47], while citronellal (83.5%) was found as the predominant compound in the EO from the collected plant from Benin [48]. The EO of E. citriodora, which yielded 1.73%, showed strong inhibition for the radial growth of F. oxysporum f. sp. lycopersici, Alternaria triticina, Rhizoctonia solani, Macrophomina phaseolina, Colletotrichum lindemuthianum, Helminthosporium oryzae, and Alternaria solani [49]. The Algerian E. citriodora leaf EO showed the presence of dominant compounds citronellal (69.77%), citronellol (10.63%), and isopulegol (4.66%) with potent antifungal activity against Microsporum canis, Trichophyton mentagrophytes, and T. rubrum [50].
E. citriodora were citronellal (40%), isopulegol (14.6%), and citronellol (13%) grown in Colombia [51]. With percentages of 61.78%, 15.54%, and 7.90%, citronellal, isopulegol, and -citronellol were the principal components of the EO of C. citriodora. The EO and citronellal had a significant negative impact on the growth of Pyricularia grisea, Aspergillus spp., and Colletotrichum musae [52]. Citronellal (52.2%), citronellol (12.3%), and isopulegol (11.9%) were the three main compunds of the 0.6% EO obtained from the leaves of the lemon-scented eucalyptus (Eucalyptus citriodora) collected from the campus of the Anjab University in Chandigarh, India [53]. Citronellal (67.5%), citronellol (6.9%), and menthol (6.1%), according to a chromatographic examination of C. citriodora EO from Brazil, were the main constituents [54].
Methanolic extract of E. citriodora had the highest antimycotic efficacy against four Aspergillus spp., with a value of 14.5% followed by EO (12.9%), chloroform extract (10.15%), and aqueous extract (10%) [10]. Flavonoid glycoside, citrioside C, kaempferol-3-O-β-d-glucopyranosyl (12)-α-l-rhamnoside, kaempferol-3-O-α-l-rhamnoside, and quercetin-3-O-α-l-rhamnoside were isolated from leaves of E. citriodora [55]. 8-[1-(p-hydroxyphenyl)ethyl]rhamnocitrin, cinnamic acid, p-coumaric acid, caffeic acid (4), and gallic acid were isolated from the kino of E. citriodora [56]. The flavonoids sakuranetin, narigenin, aromadendrin 7-methyl ether, aromadendrin, kaempferol 7-methyl ether, gallic acid, the glycosides 1-O-cinnamoyl-6-O-(p-coumaroyl)-β-D-glucopyranoside, 7-methylaromadendrin-4’-O-(6’’-trans-p-coumaroyl)-β-D-glucopyranoside, 6-O-(p-coumaroyl)-D-glucopyranoside, and the tannin 1-O-2,O-digalloyl-6-O-(p-coumaroyl)-β-D-glucopyranoside were isolated from E. citriodora [11].
The main compounds found in Cupressus macrocarpa leaves’ EO included sabinene (11.94%), 4-terpinenol (11.34%), citronellol (9.59%), citronellal (9.85%), p-cymene (7.67%), spathulenol (5.24%), γ-terpinene (5.05%), camphor (4.31%), and limonene (3.2%). Neral (31–35%), hydroxy citronellal (12–16%), geraniol (3–4%), trans-piperitol (7–8%), isobornyl isobutrate (0.7–6.61%), linalool (0.6–5.21%), terpinyl acetate (0.10–3.27%), and myrcene (0.22–2.60%) were the major components in Cup. macrocarpa EOs of fresh and dried leaves [57].
From the extract of Cup. macrocarpa, several bioactive substances were isolated, including gallic acid, cupressuflavone, sandaracopimaric acid, blumenol-C-glucoside, β-sitosterol, 3-methylbut-3-en-1-ol-O-b-D-glucopyranoside, 3’-demethoxy-dihydrodehydrodiconiferyl alcohol-9’-O-a-Lrhamnopyranoside, agathadiol, protocatechuic acid, and shikimic acid, which they observed antibacterial activity against Staphylococcus aureus [58]. A leaf extract from Cup. macrocarpa was analyzed using LC–ESI–MS/MS, which revealed 49 compounds, including (-)-shikimic acid, D-(-)-quinic acid, myricetin, p-coumaric acid, rhoifolin, quercetin, kaempferol derivatives, naringenin-7-O-glucoside, and luteolin-3,7-di-O-glucoside [59]. The extract from Cup. macrocarpa contained the polyphenolic substances gallic acid, catechol, p-hydroxy benzoic acid, caffeine, chlorogenic, vanillic acid, caffeic acid, syringic acid, vanillin, p-coumaric acid, ferulic acid, benzoic acid, hyperoside rose, ellagic acid, O-coumaric acid, salicylic acid, myricetin, and quercitin [60].
Trans-β-ocimene (19.11%), α-pinene (18.79%), caryophyllene (9.30%), (Z)-β-ocimene (8.16%), and limonene (6.00%) were the primary constituents of the EO from Syzygium cumini leaf EO. Pinocarveol, α-terpeneol, myrtenol, eucarvone, muurolol, myrtenal, geranylacetone, α-cadinol, and pinocarvone were previously revealed by S. cumini EO [61]. α-Pinene, β-pinene, trans-caryophyllene, 1,3,6-octatriene, Δ-3-carene, α-caryophyllene, and α-limonene were presented as the main compounds in EO from leaves of S. cumini [62]. While another study showed that α-pinene, (Z)-β-ocimene, and (E)-β-ocimene were the main compounds [26]. The majority of the sesquiterpene components, including terpineol, α-caryophyllene, and β-caryophyllene, were identified in S. cumini EO [27].
It was demonstrated that the content of β-pinene and β-caryophyllene in the EO was associated with its antiinflammatory properties [63]. It was shown that S. cumini EO has antihyperlipidemic, antiallergic, and good antibacterial activity against bacteria, fungi, and protozoa parasites of the species Leishmania and Trypanosoma [64]. In comparison to methylene chloride extract and EO, the methanol extract showed more action against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Neisseria gonorrhoeae, Bacillus subtilis, and Enterococcus faecalis [62]. These might be connected to the methanolic extract’s increased total phenolic and flavonoid concentration when compared to other extracts. The EO of leaves from S. cumini showed moderate activity against E. coli and the main component was α-pinene (53.21%) [65].
The primary ingredient concentrations in the EOs of S. cumini, C. citriodora, and C. macrocarpa were different from those described in the literature. These variations in EO compositions could result from a variety of environmental (climatic, seasonal, or geographical) and genetic variations [66,67,68,69]. Additionally, it should be taken into account that the synergistic interactions between the various EO components may contribute to the activity of them. Previously, it was shown that the citronellal and linalool in Cymbopogon nardus EO synergized to provide a potent antifungal action [70].
The major chemical constituent citronellal may be responsible for the activity against fungal growth. Citronellal is reported to show activities against fungi from Aspergillus [70]. Citronellal was observed to have a strong effect on the growth of A. niger [71], while other compounds like α-pinene, β-pinene, myrcene, geraniol, and linalool have antifungal activity [70].
Ferulic and p-coumaric acids showed significantly enhanced inhibitory potential in a synthetic media; ferulic acid remained active against Monilinia fructicola and Alternaria alternata, and it surpassed p-coumaric acid in suppressing Botrytis cinerea [72]. The most effective antifungal agents were caffeineic, 2,3,4-trihydroxybenzoic, p-coumaric, and protocatechuic acids, which virtually completely suppressed A. alternata on both early- and late-ripening sweet cherry [73]. Additionally, p-coumaric acid completely stopped Fusarium from growing. Conversely, at the same dose of 1000 μg/g, ferulic acid inhibited 64% of Fusarium mycelial development and was especially effective against B. cinerea growth [74, 75].
5 Conclusion
This study looked into the chemical composition and antifungal properties of methanol extracts (MEs) and essential oils (EOs) derived from the leaves of three trees grown in Egypt: Corymbia citriodora, Cupressus macrocarpa, and Syzygium cumini. GC–MS was used to identify the chemical components of EOs, while HPLC was used to identify the chemical components of MEs. Trials evaluating the obtained EOs and MEs’ antifungal efficacy against Fusarium solani were conducted when applied to wood samples. For the studied EOs and MEs, distinct and significant inhibitory effects were seen. However, additional research is needed to assess the toxicity and the efficacy of treatment for long-term as antifungal agents before the acquired EOs and MLEs can be used as substitutes for synthetic fungicides.
Data availability
Not applicable.
References
Mulyaningsih S, Sporer F, Zimmermann S, Reichling J, Wink M (2010) Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 17(13):1061–1066. https://doi.org/10.1016/j.phymed.2010.06.018
Tripathi A, Sharma N, Sharma V (2009) In vitro efficacy of Hyptis suaveolens L. (Poit.) essential oil on growth and morphogenesis of Fusarium oxysporum f.sp. gladioli (Massey) Snyder & Hansen. World J Microbiol Biotechnol 25(3):503–512. https://doi.org/10.1007/s11274-008-9916-y
Shakam HM, Mohamed AA, Salem MZM (2022) Down-regulatory effect of essential oils on fungal growth and Tri4 gene expression for some Fusarium oxysporum strains: GC-MS analysis of essential oils. Arch Phytopathol Plant Prot 55(8):951–972. https://doi.org/10.1080/03235408.2022.2064081
Hu F, Tu X-F, Thakur K, Hu F, Li X-L, Zhang Y-S, Zhang J-G, Wei Z-J (2019) Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem Toxicol 134:110821. https://doi.org/10.1016/j.fct.2019.110821
Zhang Z, Mo Z, Zhang X, Wang J, Li J, Shi H, Wang P, Lin Z (2022) The antifungal activity and action mechanism of lemongrass (Cymbopogon flexuosus) essential oil against Fusarium avenaceum. J Essent Oil Bearing Plants 25(3):536–547. https://doi.org/10.1080/0972060X.2022.2090283
Hung W, Chen Z, Lee S (2018) Antioxidant and lipoxygenase inhibitory activity of the Kino of Eucalyptus citriodora. Indian J Pharma Ceutical Sci 80(5):955–959. https://doi.org/10.4172/pharmaceutical-sciences.1000444
Ali S, Chatha SAS, Ali Q, Hussain AI, Hussain SM, Perveen R (2016) Oxidative stability of cooking oil blend stabilized with leaf extract of Eucalyptus citriodora. Int J Food Prop 19(7):1556–1565. https://doi.org/10.1080/10942912.2015.1047514
Siddique YH, Mujtaba SF, Jyoti S, Naz F (2013) GC–MS analysis of Eucalyptus citriodora leaf extract and its role on the dietary supplementation in transgenic Drosophila model of Parkinson’s disease. Food Chem Toxicol 55:29–35. https://doi.org/10.1016/j.fct.2012.12.028
Salem MZM, Elansary HO, Ali HM, El-Settawy AA, Elshikh MS, Abdel-Salam EM, Skalicka-Woźniak K (2018) Bioactivity of essential oils extracted from Cupressus macrocarpa branchlets and Corymbia citriodora leaves grown in Egypt. BMC Complement Altern Med 18(1):23. https://doi.org/10.1186/s12906-018-2085-0
Javed S, Shoaib A, Mahmood Z, Mushtaq S, Iftikhar S (2012) Analysis of phytochemical constituents of Eucalyptus citriodora L. responsible for antifungal activity against post-harvest fungi. Natl Prod Res 26(18):1732–1736. https://doi.org/10.1080/14786419.2011.607451
Freitas MO, Ponte FA, Lima MAS, Silveira ER (2008) Flavonoids and triterpenes from the nest of the stingless bee Trigona spinipes. J Braz Chem Soc 19:532–535. https://doi.org/10.1590/S0103-50532008000300022
Freitas MO, Lima MAS, Silveira ER (2007) NMR assignments of unusual flavonoids from the kino of Eucalyptus citriodora. Magn Reson Chem 45(3):262–264. https://doi.org/10.1002/mrc.1929
da Cruz JER, da Costa Guerra JF, de Souza GM, Freitas GROe, Morais ER (2019) Phytochemical analysis and evaluation of antimicrobial activity of Peumus boldus, Psidium guajava, Vernonia polysphaera, Persea americana, Eucalyptus citriodora leaf extracts and Jatropha multifida raw sap. Curr Pharm Biotechnol 20(5):433–444. https://doi.org/10.2174/1389201020666190409104910
Sarma N, Gogoi R, Loying R, Begum T, Munda S, Pandey S, Lal M (2021) Phytochemical composition and biological activities of essential oils extracted from leaves and flower parts of Corymbia citriodora (Hook.). J Environ Biol 42:552–562. https://doi.org/10.22438/jeb/42/2(SI)/SI-285
Sharififar F, Mozaffarian V, Moradkhani S (2007) Comparison of antioxidant and free radical scavenging activities of the essential oils from flowers and fruits of Otostegia persica Boiss. Pak J Biol Sci: PJBS 10(21):3895–3899. https://doi.org/10.3923/pjbs.2007.3895.3899
Eckenwalder JE (1993) Cupressus. Flora of North America Editorial Committee : Flora of North America North of Mexico, Oxford University Press. Vol. 2. The Gymnosperm Data base [ONLINE Available at http://www.conifers.org. [Accessed 25 March, 2011].
Little DP (2006) Evolution and circumscription of the true cypresses (Cupressaceae: Cupressus). Syst Bot 31(3):461–480. https://doi.org/10.1600/036364406778388638
Kuiate J-R, Bessière JM, Zollo PHA, Kuate SP (2006) Chemical composition and antidermatophytic properties of volatile fractions of hexanic extract from leaves of Cupressus lusitanica Mill. from Cameroon. J Ethnopharmacol 103(2):160–165. https://doi.org/10.1016/j.jep.2005.07.022
Price S, Cert Ed, FISPA, MIFA, FIAM, Price L, Cert Ed MIT(Trichology), FISPA, FIAM and Price P (2011) Aromatherapy for health professionals, 5th edn., Elsevier E-Book, pp 560
Thukral SK, Singh S, Sharma SK (2014) Pharmacognostical standardization of leaves of Cupressus macrocarpa Hartweg. ex Gordon. J Appl Pharm Sci 4(5):071–074. https://doi.org/10.7324/JAPS.2014.40513
Carman RM, Sutherland MD (1979) Cupressene and other diterpenes of Cupressus species. Aust J Chem 32(5):1131–1142
Cool LG (2005) Sesquiterpenes from Cupressus macrocarpa foliage. Phytochemistry 66(2):249–260. https://doi.org/10.1016/j.phytochem.2004.11.002
Al-Sayed E, Gad HA, El-Shazly M, Abdel-Daim MM, Nasser Singab A (2018) Anti-inflammatory and analgesic activities of cupressuflavone from Cupressus macrocarpa: Impact on pro-inflammatory mediators. Drug Dev Res 79(1):22–28. https://doi.org/10.1002/ddr.21417
Ayyanar M, Subash-Babu P (2012) Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed 2(3):240–246. https://doi.org/10.1016/s2221-1691(12)60050-1
Shafi PM, Rosamma MK, Jamil K, Reddy PS (2002) Antibacterial activity of Syzygium cumini and Syzygium travancoricum leaf essential oils. Fitoterapia 73(5):414–416. https://doi.org/10.1016/S0367-326X(02)00131-4
Dias CN, Rodrigues KAF, Carvalho FAA, Carneiro SMP, Maia JGS, Andrade EHA, Moraes DFC (2013) Molluscicidal and leishmanicidal activity of the leaf essential oil of Syzygium cumini (L.) skeels from Brazil. Chem Biodivers 10(6):1133–1141. https://doi.org/10.1002/cbdv.201200292
Machado RRP, Jardim DF, Souza AR, Scio E, Fabri RL, Carpanez AG, Grazul RM, de Mendonça JPRF, Lesche B, Aarestrup FM (2013) The effect of essential oil of Syzygium cumini on the development of granulomatous infl ammation in mice. Rev Bras 23(3):488–496. https://doi.org/10.1590/S0102-695X2013005000030
Yang VW, Clausen CA (2007) Antifungal effect of essential oils on southern yellow pine. Int Biodeterior Biodegradation 59(4):302–306. https://doi.org/10.1016/j.ibiod.2006.09.004
Mohareb ASO, Badawy MEI, Abdelgaleil SAM (2013) Antifungal activity of essential oils isolated from Egyptian plants against wood decay fungi. J Wood Sci 59(6):499–505. https://doi.org/10.1007/s10086-013-1361-3
Russo R, Palla F (2023) Plant essential oils as biocides in sustainable strategies for the conservation of cultural heritage. Sustainability 15(11):8522. https://doi.org/10.3390/su15118522
Batish DR, Singh HP, Kohli RK, Kaur S (2008) Eucalyptus essential oil as a natural pesticide. For Ecol Manage 256(12):2166–2174. https://doi.org/10.1016/j.foreco.2008.08.008
Yang D-Q (2009) Potential utilization of plant and fungal extracts for wood protection. For Prod J 59(4):97–104
Yingprasert W, Matan N, Matan N (2015) Effects of surface treatment with cinnamon oil and clove oil on mold resistance and physical properties of rubberwood particleboards. Eur J Wood Wood Prod 73(1):103–109. https://doi.org/10.1007/s00107-014-0857-x
Vettraino AM, Zikeli F, Humar M, Biscontri M, Bergamasco S, Romagnoli M (2023) Essential oils from Thymus spp. as natural biocide against common brown- and white-rot fungi in degradation of wood products: antifungal activity evaluation by in vitro and FTIR analysis. Eur J Wood Wood Prod 81(3):747–763. https://doi.org/10.1007/s00107-022-01914-3
Woźniak M, Kwaśniewska-Sip P, Waśkiewicz A, Cofta G, Ratajczak I (2020) The possibility of propolis extract application in wood protection. Forests 11(4):465. https://doi.org/10.3390/molecules27196392
Chakravarty I, Parmar VM, Mandavgane SA (2021) Current trends in essential oil (EO) production. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01963-3
Ahmed YM, Gaber MM, Hafez RI, El-Adawy AM, Mostafa AMA (2021) Effect of Moringa oleifera seeds extract on Tetranychus urticae (Koch). J Appl Plant Prot 10(1):77–81. https://doi.org/10.21608/japp.2021.234780
Mohamed AA, El-Hefny M, El-Shanhorey NA, Ali HM (2020) Foliar application of bio-stimulants enhancing the production and the toxicity of Origanum majorana essential oils against four rice seed-borne fungi. Molecules 25(10):2363. https://doi.org/10.3390/molecules25102363
Taha AS, Ibrahim IHM, Abo-Elgat WAA, Abdel-Megeed A, Salem MZM, El-Kareem MSMA (2023) GC–MS, quantum mechanics calculation and the antifungal activity of river red gum essential oil when applied to four natural textiles. Sci Rep 13(1):18214. https://doi.org/10.1038/s41598-023-45480-x
Stein SE (2014) NIST mass spectral search program version 2.2 and NIST/EPA/NIH Mass Spectral Library. (National Institute of Standards and Technology, US Secretary of Commerce, USA). https://www.nist.gov/system/files/documents/srd/NIST1aVer22Man.pdf. Accessed June 2014
Elbanoby NE, El-Settawy AAA, Mohamed AA, Salem MZM (2022) Phytochemicals derived from Leucaena leucocephala (Lam.) de Wit (Fabaceae) biomass and their antimicrobial and antioxidant activities: HPLC analysis of extracts. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03420-1
El-Hefny M, Mohamed AA, Abdelkhalek A, Salem MZM (2023) Productivity and phytochemicals of Asclepias curassavica in response to compost and silver nanoparticles application: HPLC analysis and antibacterial activity of extracts. Plants 12:2274. https://doi.org/10.3390/plants12122274
Tsoumis G (2013) Wood as raw material: source, structure, chemical composition, growth, degradation and identification, Elsevier; 2013 Oct 22, PP. 288. Elsevier
Correa-Pacheco ZN, Bautista-Baños S, Valle-Marquina MÁ, Hernández-López M (2017) The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on Colletotrichum gloeosporioides growth in vitro and on cv Hass avocado and fruit quality. J Phytopathol 165(5):297–305. https://doi.org/10.1111/jph.12562
CoStat V (1999) 6, 303 Copyright (1998–2004). CoHort Software 798
Snedecor GW, Cochran WG (1994) Statistical methods, 8th edn. IOWA State University Press, Ames, Iowa, USA
Luís Â, Duarte AP, Pereira L, Domingues F (2017) Chemical profiling and evaluation of antioxidant and anti-microbial properties of selected commercial essential oils: a comparative study. Medicines 4(2):36
Gbenou JD, Ahounou JF, Akakpo HB, Laleye A, Yayi E, Gbaguidi F, Baba-Moussa L, Darboux R, Dansou P, Moudachirou M, Kotchoni SO (2013) Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Mol Biol Rep 40(2):1127–1134. https://doi.org/10.1007/s11033-012-2155-1
Ramezani H, Singh HP, Batish DR, Kohli RK (2002) Antifungal activity of the volatile oil of Eucalyptus citriodora. Fitoterapia 73(3):261–262. https://doi.org/10.1016/S0367-326X(02)00065-5
Tolba H, Moghrani H, Benelmouffok A, Kellou D, Maachi R (2015) Essential oil of Algerian Eucalyptus citriodora: chemical composition, antifungal activity. J Mycol Méd 25(4):e128–e133. https://doi.org/10.1016/j.mycmed.2015.10.009
Olivero-Verbel J, Nerio LS, Stashenko EE (2010) Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. Pest Manag Sci 66(6):664–668. https://doi.org/10.1002/ps.1927
Aguiar RWdS, Ootani MA, Ascencio SD, Ferreira TPS, Santos MMd, Santos GRd (2014) Fumigant antifungal activity of Corymbia citriodora and Cymbopogon nardus essential oils and citronellal against three fungal species. Sci World J 2014:492138. https://doi.org/10.1155/2014/492138
Batish DR, Singh HP, Setia N, Kaur S, Kohli RK (2006) Chemical composition and inhibitory activity of essential oil from decaying leaves of Eucalyptus citriodora. 61(1-2):52-56. https://doi.org/10.1515/znc-2006-1-210
Ribeiro JC, Ribeiro WLC, Camurça-Vasconcelos ALF, Macedo ITF, Santos JML, Paula HCB, Araújo Filho JV, Magalhães RD, Bevilaqua CML (2014) Efficacy of free and nanoencapsulated Eucalyptus citriodora essential oils on sheep gastrointestinal nematodes and toxicity for mice. Vet Parasitol 204(3):243–248. https://doi.org/10.1016/j.vetpar.2014.05.026
Zhou Z-L, Yin W-Q, Zou X-P, Huang D-Y, Zhou C-L, Li L-M, Chen K-C, Guo Z-Y, Lin S-Q (2014) Flavonoid glycosides and potential antivirus activity of isolated compounds from the leaves of Eucalyptus citriodora. J Korean Soc Appl Biol Chem 57(6):813–817. https://doi.org/10.1007/s13765-014-4264-0
Lee S-W (2019) A new lipoxygenase inhibitory flavonoid from the kino of Eucalyptus citriodora. Chem Nat Compd 55(1):18–20. https://doi.org/10.1007/s10600-019-02655-1
El-Ghorab AH, El-Massry KF, Shaaban HA (2007) Effect of drying on the chemical composition of the Egyptian Cupressus macrocarpa (Hartw. ex Gordon) essential oils and their biological characteristics. J Essent Oil Bearing Plants 10(5):399–411. https://doi.org/10.1080/0972060X.2007.10643573
Harraz FM, Hammoda HM, El-Hawiet A, Radwan MM, Wanas AS, Eid AM, ElSohly MA (2020) Chemical constituents, antibacterial and acetylcholine esterase inhibitory activity of Cupressus macrocarpa leaves. Nat Prod Res 34(6):816–822. https://doi.org/10.1080/14786419.2018.1508140
Attallah NG, Negm WA, Elekhnawy E, Elmongy EI, Altwaijry N, El-Haroun H, El-Masry TA, El-Sherbeni SA (2021) Elucidation of phytochemical content of Cupressus macrocarpa leaves: in vitro and in vivo antibacterial effect against methicillin-resistant Staphylococcus aureus clinical isolates. Antibiotics 10(8):890. https://doi.org/10.3390/antibiotics10080890
ElSayed AI, Rafudeen MS, Ganie SA, Hossain MS, Gomaa AM (2022) Seed priming with cypress leaf extract enhances photosynthesis and antioxidative defense in zucchini seedlings under salt stress. Sci Hortic 293:110707. https://doi.org/10.1016/j.scienta.2021.110707
Jirovets L, Buchbauer G, Puschmann C, Fleischhacker W, Shafi P, Rosamma M (1999) Analysis of the essential oil of the fresh leaves of Syzygium cumini and Syzygium travancoricoricum from South India. J Essent Oil Bearing Plant 2:68–77
Mohamed AA, Ali SI, El-Baz FK (2013) Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini leaves. PLoS One 8(4):e60269. https://doi.org/10.1371/journal.pone.0060269
Siani AC, Souza MC, Henriques MGMO, Ramos MFS (2013) Anti-inflammatory activity of essential oils from Syzygium cumini and Psidium guajava. Pharm Biol 51(7):881–887. https://doi.org/10.3109/13880209.2013.768675
Rodrigues KAdF, Amorim LV, Dias CN, Moraes DFC, Carneiro SMP, Carvalho FAdA (2015) Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J Ethnopharmacol 160:32–40. https://doi.org/10.1016/j.jep.2014.11.024
Dias CN, Rodrigues KA, Carvalho FA, Carneiro SM, Maia JG, Andrade EH, Moraes DF (2013) Molluscicidal and leishmanicidal activity of the leaf essential oil of Syzygium cumini (L.) SKEELS from Brazil. Chem Biodivers 10(6):1133–1141. https://doi.org/10.3390/molecules27103281
Andrade MA, Cardoso MdG, Batista LR, Mallet ACT, Machado SMF (2012) Essential oils of Cinnamomum zeylanicum, Cymbopogon nardus and Zingiber officinale: composition, antioxidant and antibacterial activities. Rev Ciênc Agron 43:399–408. https://doi.org/10.1590/S1806-66902012000200025
Lee Y-S, Kim J, Shin S-C, Lee S-G, Park I-K (2008) Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flavour Fragr J 23(1):23–28. https://doi.org/10.1002/ffj.1850
Perry NB, Anderson RE, Brennan NJ, Douglas MH, Heaney AJ, McGimpsey JA, Smallfield BM (1999) Essential oils from Dalmatian sage (Salvia officinalis L.): variations among individuals, plant parts, seasons, and sites. J Agric Food Chem 47(5):2048–2054. https://doi.org/10.1021/jf981170m
Abdelsalam NR, Salem MZM, Ali HM, Mackled MI, El-Hefny M, Elshikh MS, Hatamleh AA (2019) Morphological, biochemical, molecular, and oil toxicity properties of Taxodium trees from different locations. Ind Crops Prod 139:111515. https://doi.org/10.1016/j.indcrop.2019.111515
Nakahara K, Alzoreky NS, Yoshihashi T, Nguyen HTT, Trakoontivakorn G (2013) Chemical composition and antifungal activity of essential oil from Cymbopogon nardus (Citronella Grass). Japan Agric Res Q: JARQ 37(4):249–252. https://doi.org/10.6090/jarq.37.249
Kim E, Park I-K (2012) Fumigant antifungal activity of Myrtaceae essential oils and constituents from Leptospermum petersonii against three Aspergillus species. Molecules 17(9):10459–10469. https://doi.org/10.3390/molecules170910459
Hernández A, Ruiz-Moyano S, Galván AI, Merchán AV, Pérez Nevado F, Aranda E, Serradilla MJ, Córdoba MdG, Martín A (2021) Anti-fungal activity of phenolic sweet orange peel extract for controlling fungi responsible for post-harvest fruit decay. Fungal Biol 125(2):143–152. https://doi.org/10.1016/j.funbio.2020.05.005
Wang M, Jiang N, Wang Y, Jiang D, Feng X (2017) Characterization of phenolic compounds from early and late ripening sweet cherries and their antioxidant and antifungal activities. J Agric Food Chem 65(26):5413–5420. https://doi.org/10.1021/acs.jafc.7b01409
Barral B, Chillet M, Minier J, Léchaudel M, Schorr-Galindo S (2017) Evaluating the response to Fusarium ananatum inoculation and antifungal activity of phenolic acids in pineapple. Fungal Biol 121(12):1045–1053. https://doi.org/10.1016/j.funbio.2017.09.002
Patzke H, Schieber A (2018) Growth-inhibitory activity of phenolic compounds applied in an emulsifiable concentrate - ferulic acid as a natural pesticide against Botrytis cinerea. Food Res Int 113:18–23. https://doi.org/10.1016/j.foodres.2018.06.062
Acknowledgements
This work was from an undergraduate research project based upon work supported by the Department of Forestry and Wood Technology, Faculty of Agriculture, Alexandria University, Alexandria, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors have equal contribution in conceptualization, investigation, data curation, methodology, software, and writing (original draft) of the manuscript. All authors: writing—review, edit, read, and approve the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohareb, A.S.O., Elashmawy, M.A.A., Nawar, M.E.M. et al. Chemical compositions and antifungal activity of Corymbia citriodora, Cupressus macrocarpa, and Syzygium cumini extracts: GC–MS and HPLC analysis of essential oils and phenolic compounds. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05106-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05106-8